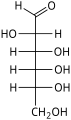
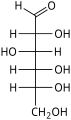
Aldohexose
An aldohexose is a hexose with an aldehyde group on one end.[1][2]
The aldohexoses have four chiral centres for a total of 16 possible aldohexose stereoisomers (24). Of these, only three commonly occur in nature: D-glucose, D-galactose, and D-mannose. The D/L configuration is based on the orientation of the hydroxyl at position 5, and does not refer to the direction of optical activity.
There are eight D-aldohexoses as shown, and eight L-aldohexoses which are their enantiomers or mirror images.
The chemist Emil Fischer is said to have devised the following mnemonic device for remembering the order given above, which corresponds to the configurations about the chiral centers when ordered as 3-bit binary strings: All altruists gladly make gum in gallon tanks.
Deoxyaldohexoses
Aldohexoses can have one or more of their hydroxyl groups replaced by hydrogens to form deoxyaldohexoses. The following are well known cases of such compounds:
- L-Fucose (6-deoxy-L-galactose)
- L-Rhamnose (6-deoxy-L-mannose)
- D-Quinovose (6-deoxy-D-glucose), found as part of the sulfolipid sulfoquinovosyl diacylglycerol (SQDG)
- L-Pneumose (6-deoxy-L-talose)
References
- ^ Thisbe K. Lindhorst (2007). Essentials of Carbohydrate Chemistry and Biochemistry (1 ed.). Wiley-VCH. ISBN 3-527-31528-4.
- ^ John F. Robyt (1997). Essentials of Carbohydrate Chemistry (1 ed.). Springer. ISBN 0-387-94951-8.
External links
 Media related to Aldohexoses at Wikimedia Commons
Media related to Aldohexoses at Wikimedia Commons