Boron trifluoride etherate
Appearance
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.355 |
PubChem CID
|
|
| UNII | |
| UN number | 2604 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10BF3O | |
| Molar mass | 141.93 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.15 g cm–3 |
| Boiling point | 126 °C (259 °F; 399 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
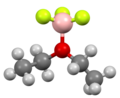
Boron trifluoride etherate, strictly boron trifluoride diethyl etherate, or boron trifluoride–ether complex, is the chemical compound with the formula BF3O(C2H5)2, often abbreviated BF3OEt2. It is a colorless liquid, although older samples can appear brown. The compound is used as a source of boron trifluoride in many chemical reactions that require a Lewis acid.[1] The compound features tetrahedral boron coordinated to a diethylether ligand.[2] Many analogues are known, including the methanol complex.
-
Structure of the etherate of BF3
-
Structure of the etherate of BF3, viewed down O-B bond
Reactions
It serves as a source of boron trifluoride via the equilibrium:
- BF3OEt2 BF3 + OEt2
The BF3 binds to even weak Lewis bases, inducing reactions of the resulting adducts with nucleophiles..[1]
References
- ^ a b Veronica Cornel; Carl J. Lovely (2007). "Boron Trifluoride Etherate". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rb249.pub2.
{{cite encyclopedia}}: Cite has empty unknown parameter:|1=(help) - ^ "Synthesis, Molecular Structure, and EPR Analysis of the Three-Coordinate Ni(I) Complex [Ni(PPh3)3][BF4]". J. Phys. Chem. A. 112: 12449–12455. 2008. doi:10.1021/jp802462x.
{{cite journal}}: Unknown parameter|authors=ignored (help)