Calcitriol
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | US: /ˌkælsɪˈtraɪɒl/;[1][2][3][4][5] UK: /kælˈsɪtriɒl/ |
| Trade names | Rocaltrol, Calcijex, Decostriol |
| MedlinePlus | a682335 |
| Pregnancy category |
|
| Routes of administration | Oral, IV, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Renal |
| Elimination half-life | 5–8 hours (adults), 27 hours (children) |
| Excretion | Faeces (50%), urine (16%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.315 |
| Chemical and physical data | |
| Formula | C27H44O3 |
| Molar mass | 416.64 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Calcitriol (INN), also called 1,25-dihydroxycholecalciferol, or 1alpha,25-dihydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and other variants, is the hormonally active metabolite of vitamin D which has three hydroxyl groups. It can be abbreviated 1α,25-(OH)2D3 or simply 1,25(OH)2D.[6] Calcitriol increases the level of calcium (Ca2+) in the blood by increasing the uptake of calcium from the gut into the blood, increasing reabsorption of calcium by the kidneys, and possibly increasing the release of calcium into the blood from bone.[7]
Nomenclature
Calcitriol usually refers specifically to 1,25-dihydroxycholecalciferol. Because cholecalciferol already has one hydroxyl group, only two (1,25) are further specified in this nomenclature, but there are three (1,3,25-triol), as indicated in when calcitriol is used. The 1-hydroxy group is in the alpha position, and this may be specified in the name, for instance in the abbreviation 1α,25-(OH)2D3.[6]
Calcitriol is the strictly the 1-hydroxylation product of calcifediol (25-OH vitamin D3), derived from cholecalciferol (vitamin D3), rather than the product of hydroxylations of ergocalciferol (vitamin D2).[6] 1α,25-Dihydroxyergocalciferol (ercalcitriol) should be used for the vitamin D2 product.[6] However, the terminology of 1,25-dihydroxyvitamin D, or 1,25(OH)2D, is often used to refer to both types of active forms of vitamin D. Indeed, both bind to the vitamin D receptor and produce biological effects.[8] In clinical use, the differences are unlikely to have major importance.[9]
Calcitriol is marketed as a pharmaceutical for medical use under various trade names including Rocaltrol (Roche), Calcijex (Abbott), Decostriol (Mibe, Jesalis), Vectical (Galderma), and Rolsical (Sun Pharma).
Function
Calcitriol increases blood calcium levels ([Ca2+
]) by:
- Promoting absorption of dietary calcium from the gastrointestinal tract.
- Increasing renal tubular reabsorption of calcium, thus reducing the loss of calcium in the urine.
- Stimulating release of calcium from bone. For this it acts on the specific type of bone cells referred to as osteoblasts, causing them to release RANKL, which in turn activates osteoclasts.[10]
Calcitriol acts in concert with parathyroid hormone (PTH) in all three of these roles. For instance, PTH also indirectly stimulates osteoclasts. However, the main effect of PTH is to increase the rate at which the kidneys excrete inorganic phosphate (Pi), the counterion of Ca2+
. The resulting decrease in serum phosphate causes hydroxyapatite (Ca5(PO4)3OH) to dissolve out of bone thus increasing serum calcium. PTH also stimulates the production of calcitriol (see below).[7]
Many of the effects of calcitriol are mediated by its interaction with the calcitriol receptor, also called the vitamin D receptor or VDR. For instance, the unbound inactive form of the calcitriol receptor in intestinal epithelial cells resides in the cytoplasm. When calcitriol binds to the receptor, the ligand-receptor complex translocates to the cell nucleus, where it acts as a transcription factor promoting the expression of a gene encoding a calcium binding protein. The levels of the calcium binding protein increase enabling the cells to actively transport more calcium (Ca2+
) from the intestine across the intestinal mucosa into the blood.[7]
The maintenance of electroneutrality requires that the transport of Ca2+
ions catalyzed by the intestinal epithelial cells be accompanied by counterions, primarily inorganic phosphate. Thus calcitriol also stimulates the intestinal absorption of phosphate.[7]
The observation that calcitriol stimulates the release of calcium from bone seems contradictory, given that sufficient levels of serum calcitriol generally prevent overall loss of calcium from bone. It is believed that the increased levels of serum calcium resulting from calcitriol-stimulated intestinal uptake causes bone to take up more calcium than it loses by hormonal stimulation of osteoclasts.[7] Only when there are conditions, such as dietary calcium deficiency or defects in intestinal transport, which result in a reduction of serum calcium does an overall loss of calcium from bone occur.
Calcitriol also inhibits the release of calcitonin,[citation needed] a hormone which reduces blood calcium primarily by inhibiting calcium release from bone.[7] (The effect of calcitonin on renal excretion is disputed.)[11]
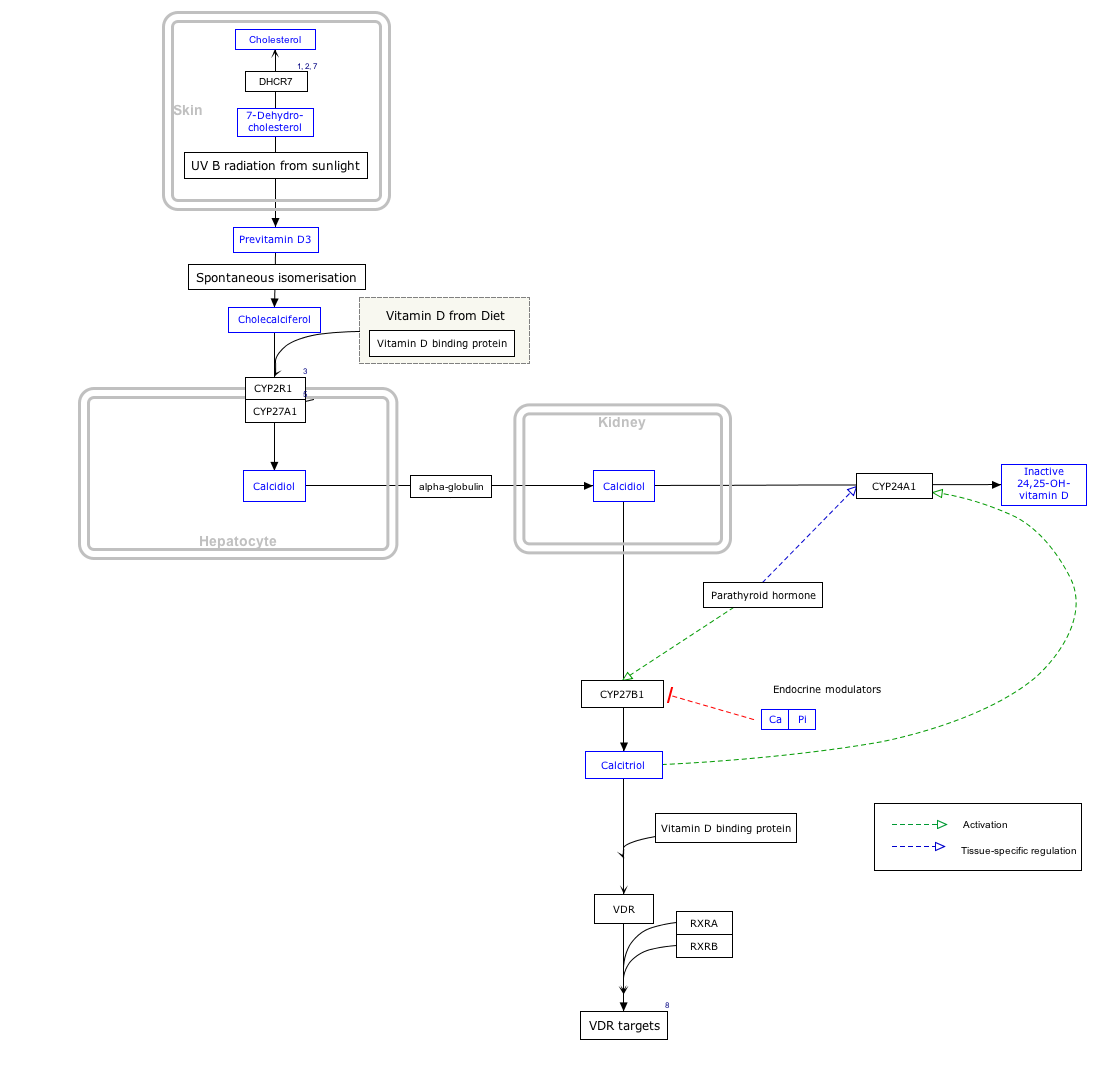
Biosynthesis and its regulation
Calcitriol is produced in the cells of the proximal tubule of the nephron in the kidneys by the action of 25-hydroxyvitamin D3 1-alpha-hydroxylase, a mitochondrial oxygenase and an enzyme which catalyzes the hydroxylation of 25-hydroxycholecalciferol (calcifediol) in the 1-alpha position.
The activity of this enzyme is stimulated by PTH. This is an important control point in Ca2+ homeostasis.[7] Additional effects on the production of calcitriol include an increase by prolactin, a hormone which stimulates lactogenesis (the formation of milk in mammary glands), a process which requires large amounts of calcium.[12] Activity is also decreased by high levels of serum phosphate and by an increase in the production of the hormone FGF23 by osteocyte cells in bone.[13]
Calcitriol is also produced outside the kidney in small amounts by many other tissues including placenta and activated macrophages.[14]
When the drug alfacalcidol is used, 25-hydroxylation in the liver will produce calcitriol as the active metabolite. This will produce greater effects than other vitamin D precursors in patients with kidney disease who have loss of the renal 1-alpha-hydroxylase.[9]
Metabolism
Calcitriol's lifespan in the body is measured in hours, unlike its precursor calcifediol whose lifespan is measured in weeks.[15] Calcitriol is inactivated by further hydroxylation to form 1,24,25-trihydroxyvitamin D, calcitroic acid. This occurs through the action of the CYP24A1 24-hydroxylase.[16] Calcitroic acid is more soluble in water and is excreted in bile and urine.
Medical use
Calcitriol is prescribed for:[17]
- Treatment of hypocalcaemia – hypoparathyroidism, osteomalacia (adults), rickets (infants, children), renal osteodystrophy, chronic kidney disease
- Treatment of osteoporosis
- Prevention of corticosteroid-induced osteoporosis
Calcitriol has been used in an ointment for the treatment of psoriasis,[18] although the vitamin D analogue calcipotriol (calcipotriene) is more commonly used.[19] Calcitriol has also been given by mouth for the treatment of psoriasis[20] and psoriatic arthritis.[21] Research on the noncalcemic actions of calcitriol and other VDR-ligand analogs and their possible therapeutic applications has been reviewed.[22]
Adverse effects
The main adverse drug reaction associated with calcitriol therapy is hypercalcemia – early symptoms include: nausea, vomiting, constipation, anorexia, apathy, headache, thirst, pruritus, sweating, and/or polyuria. Compared to other vitamin D compounds in clinical use (cholecalciferol, ergocalciferol), calcitriol has a higher risk of inducing hypercalcemia. However, such episodes may be shorter and easier to treat due to its relatively short half-life.[17]
History
It was first identified in 1971 by Michael F. Holick working in the laboratory of Hector DeLuca,[23][24] and also by Tony Norman and colleagues.[25]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Additional images
-
Calcitriol synthesis
See also
References
- ^ Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier.
- ^ Wolters Kluwer, Stedman's Medical Dictionary, Wolters Kluwer.
- ^ Merriam-Webster, Merriam-Webster's Medical Dictionary, Merriam-Webster.
- ^ Houghton Mifflin Harcourt, The American Heritage Dictionary of the English Language, Houghton Mifflin Harcourt.
- ^ Merriam-Webster, Merriam-Webster's Unabridged Dictionary, Merriam-Webster.
- ^ a b c d "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN): Nomenclature of vitamin D. Recommendations 1981". European Journal of Biochemistry. 124 (2): 223–7. 17 May 1982. doi:10.1111/j.1432-1033.1982.tb06581.x. PMID 7094913.
- ^ a b c d e f g Voet, Donald; Voet, Judith G. (2004). "Biomolecules, mechanisms of enzyme action, and metabolism". Biochemistry. Vol. 1 (3rd ed.). Wiley. pp. 663–4. ISBN 0-471-25090-2.
- ^ Cantorna, MT; Snyder, L; Lin, YD; Yang, L (22 April 2015). "Vitamin D and 1,25(OH)2D regulation of T cells (review)". Nutrients. 7 (4): 3011–21. doi:10.3390/nu7043011. PMC 4425186. PMID 25912039.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Mazzaferro, S; Goldsmith, D; Larsson, TE; Massy, ZA; Cozzolino, M (March 2014). "Vitamin D metabolites and/or analogs: which D for which patient?". Current Vascular Pharmacology. 12 (2): 339–49. doi:10.2174/15701611113119990024. PMID 23713876.
- ^ Bringhurst, F.R.; Demay, Marie B.; Krane, Stephen M.; Kronenberg, Henry M. (2008). "Ch. 346: Bone and Mineral Metabolism in Health and Disease". In Fauci, Anthony S.; Braunwald, E.; Kasper, D.L.; Hauser, S.L.; Longo, D.L.; Jameson, J.L.; Loscalzo, J. (eds.). Harrison's Principles of Internal Medicine (17th ed.). McGraw-Hill. ISBN 978-0-07-159991-7.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Carney SL (1997). "Calcitonin and human renal calcium and electrolyte transport". Miner Electrolyte Metab. 23 (1): 43–7. PMID 9058369.
- ^ Ajibade, DV; Dhawan, P; Fechner, AJ; Meyer, MB; Pike, JW; Christakos, S (July 2010). "Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin". Endocrinology. 151 (7): 2974–84. doi:10.1210/en.2010-0033. PMC 2903940. PMID 20463051.
- ^ Rodríguez-Ortiz, ME; Rodríguez, M (2015). "FGF23 as a calciotropic hormone". F1000Research. 4. doi:10.12688/f1000research.7189.1. PMC 4815615. PMID 27081473.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Adams, JS; Hewison, M (2012). "Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase". Archives of Biochemistry and Biophysics. 523 (1): 95–102. doi:10.1016/j.abb.2012.02.016. PMC 3361592. PMID 22446158.
- ^ Brandi, M.L. (2010). "Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes". Clinical Cases in Mineral and Bone Metabolism. 7 (3): 243–250. ISSN 1724-8914. PMC 3213838. PMID 22460535.
- ^ Jones, G; Prosser, DE; Kaufmann, M (January 2014). "Cytochrome P450-mediated metabolism of vitamin D." Journal of Lipid Research. 55 (1): 13–31. doi:10.1194/jlr.R031534. PMC 3927478. PMID 23564710.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Rossi, S, ed. (2006). Australian Medicines Handbook. Adelaide. ISBN 0-9757919-2-3.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Kircik, L (August 2009). "Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis (review)". Journal of Drugs in Dermatology. 8 (8 Suppl): s9–16. PMID 19702031.
- ^ Kin, KC; Hill, D; Feldman, SR (June 2016). "Calcipotriene and betamethasone dipropionate for the topical treatment of plaque psoriasis". Expert review of clinical pharmacology. 9 (6): 789–97. doi:10.1080/17512433.2016.1179574. PMID 27089906.
- ^ Smith, E. L.; Pincus, S. H.; Donovan, L.; Holick, M. F. (1988). "A novel approach for the evaluation and treatment of psoriasis". Journal of the American Academy of Dermatology. 19 (3): 516–528. doi:10.1016/S0190-9622(88)70207-8. PMID 2459166.
- ^ Huckins, D.; Felson, D. T.; Holick, M. (1990). "Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study". Arthritis & Rheumatism. 33 (11): 1723. doi:10.1002/art.1780331117. PMID 2242069.
- ^ Nagpal, S.; Na, S.; Rathnachalam, R. (2005). "Noncalcemic Actions of Vitamin D Receptor Ligands". Endocrine Reviews. 26 (5): 662–687. doi:10.1210/er.2004-0002. PMID 15798098..
- ^ Holick, MF; Schnoes, HK; Deluca, HF; Suda, T; Cousins, RJ (1971). "Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine". Biochemistry. 10 (14): 2799–804. doi:10.1021/bi00790a023. PMID 4326883.
- ^ Holick MF, Schnoes HK, DeLuca HF (April 1971). "Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine". Proceedings of the National Academy of Sciences of the United States of America. 68 (4): 803–4. Bibcode:1971PNAS...68..803H. doi:10.1073/pnas.68.4.803. PMC 389047. PMID 4323790.
- ^ Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popják G (July 1971). "1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine". Science. 173 (3991): 51–4. Bibcode:1971Sci...173...51N. doi:10.1126/science.173.3991.51. PMID 4325863.