Fuculose
Appearance
| |

| |
| Names | |
|---|---|
| IUPAC name
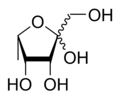
(3R,4S,5S)-2-(Hydroxymethyl)-5-methyltetrahydrofuran-2,3,4-triol
| |
| Other names
6-Deoxy-l-tagatose
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.16 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fuculose or 6-deoxy-tagatose is a ketohexose deoxy sugar.[1][2] Fuculose is involved in the process of sugar metabolism.[3] l-Fuculose can be formed from l-fucose by l-fucose isomerase and converted to L-fuculose-1-phosphate by l-fuculose kinase.[4]
See also
References
- ^ Lindhorst TK (2007). Essentials of Carbohydrate Chemistry and Biochemistry (1 ed.). Wiley-VCH. ISBN 3-527-31528-4.
- ^ Robyt JF (1997). Essentials of Carbohydrate Chemistry (1st ed.). Springer. ISBN 0-387-94951-8.
- ^ Wen L, Zang L, Huang K, Li S, Wang R, Wang PG (February 2016). "Efficient enzymatic synthesis of L-rhamnulose and L-fuculose". Bioorganic & Medicinal Chemistry Letters. 26 (3): 969–972. doi:10.1016/j.bmcl.2015.12.051. PMC 5984655. PMID 26778148.
- ^ Iqbal, Muhammad Waheed; et al. (2021). "A review on selective l-fucose/d-arabinose isomerases for biocatalytic production of l-fuculose/d-ribulose". International Journal of Biological Macromolecules. 168: 558–571. PMID 33296692.
