Lithium cobalt oxide
 | |
| |
| Names | |
|---|---|
| IUPAC name
lithium cobalt(III) oxide
| |
| Other names
lithium cobaltite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.135 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| LiCoO 2 | |
| Molar mass | 97.87 g mol−1 |
| Appearance | dark blue or bluish-gray crystalline solid |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
harmful |
| GHS labelling: | |
 
| |
| Danger | |
| H317, H350, H360 | |
| P201, P202, P261, P272, P280, P281, P302+P352, P308+P313, P321, P333+P313, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium cobalt oxide, sometimes called lithium cobaltate[2] or lithium cobaltite,[3] is a chemical compound with formula LiCoO
2. The cobalt atoms are formally in the +3 oxidation state, hence the IUPAC name lithium cobalt(III) oxide.
Lithium cobalt oxide is a dark blue or bluish-gray crystalline solid,[4] and is commonly used in the positive electrodes of lithium-ion batteries.
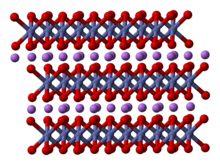
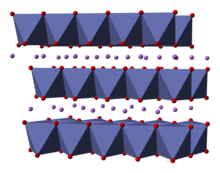
Structure
The structure of LiCoO
2 has been studied with numerous techniques including x-ray diffraction, electron microscopy, neutron powder diffraction, and EXAFS.[5]
The solid consists of layers of monovalent lithium cations (Li+
) that lie between extended anionic sheets of cobalt and oxygen atoms, arranged as edge-sharing octahedra, with two faces parallel to the sheet plane.[6] The cobalt atoms are formally in the trivalent oxidation state (Co3+
) and are sandwiched between two layers of oxygen atoms (O2−
).
In each layer (cobalt, oxygen, or lithium), the atoms are arranged in a regular triangular lattice. The lattices are offset so that the lithium atoms are farthest from the cobalt atoms, and the structure repeats in the direction perpendicular to the planes every three cobalt (or lithium) layers. The point group symmetry is in Hermann-Mauguin notation, signifying a unit cell with threefold improper rotational symmetry and a mirror plane. The threefold rotational axis (which is normal to the layers) is termed improper because the triangles of oxygen (being on opposite sides of each octahedron) are anti-aligned.[7]
Preparation
Fully reduced lithium cobalt oxide can be prepared by heating a stoichiometric mixture of lithium carbonate Li
2CO
3 and cobalt(II,III) oxide Co
3O
4 or metallic cobalt at 600–800 °C, then annealing the product at 900 °C for many hours, all under an oxygen atmosphere.[6][3][7]

Nanometer-size particles more suitable for cathode use can also be obtained by calcination of hydrated cobalt oxalate β-CoC
2O
4·2H
2O, in the form of rod-like crystals about 8 μm long and 0.4 μm wide, with lithium hydroxide LiOH, up to 750–900 °C.[9]
A third method uses lithium acetate, cobalt acetate, and citric acid in equal molar amounts, in water solution. Heating at 80 °C turns the mixture into a viscous transparent gel. The dried gel is then ground and heated gradually to 550 °C.[10]
Use in rechargeable batteries
The usefulness of lithium cobalt oxide as an intercalation electrode was discovered in 1980 by an Oxford University research group led by John B. Goodenough and Tokyo University's Koichi Mizushima.[11]
The compound is now used as the cathode in some rechargeable lithium-ion batteries, with particle sizes ranging from nanometers to micrometers.[10][9] During charging, the cobalt is partially oxidized to the +4 state, with some lithium ions moving to the electrolyte, resulting in a range of compounds Li
xCoO
2 with 0 < x < 1.[3]
Batteries produced with LiCoO
2 cathodes have very stable capacities, but have lower capacities and power than those with cathodes based on (especially nickel-rich) nickel-cobalt-aluminum (NCA) or nickel-cobalt-manganese (NCM) oxides.[12] Issues with thermal stability are better for LiCoO
2 cathodes than other nickel-rich chemistries although not significantly. This makes LiCoO
2 batteries susceptible to thermal runaway in cases of abuse such as high temperature operation (>130 °C) or overcharging. At elevated temperatures, LiCoO
2 decomposition generates oxygen, which then reacts with the organic electrolyte of the cell. This is a safety concern due to the magnitude of this highly exothermic reaction, which can spread to adjacent cells or ignite nearby combustible material.[13] In general, this is seen for many lithium ion battery cathodes.
See also
References
- ^ 442704 - Lithium cobalt(III) oxide (2012-09-14). "Sigma-Aldrich product page". Sigmaaldrich.com. Retrieved 2013-01-21.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ^ A. L. Emelina, M. A. Bykov, M. L. Kovba, B. M. Senyavin, E. V. Golubina (2011), "Thermochemical properties of lithium cobaltate". Russian Journal of Physical Chemistry, volume 85, issue 3, pages 357–363; doi:10.1134/S0036024411030071
- ^ a b c Ondřej Jankovský, Jan Kovařík, Jindřich Leitner, Květoslav Růžička, David Sedmidubský (2016) "Thermodynamic properties of stoichiometric lithium cobaltite LiCoO2". Thermochimica Acta, volume 634, pages 26-30. doi:10.1016/j.tca.2016.04.018
- ^ LinYi Gelon New Battery Materials Co., Ltd, "Lithium Cobalt Oxide (LiCoO2) for lithium ion battery ". Catalog entry, accessed on 2018-04-10,
- ^ I. Nakai; K. Takahashi; Y. Shiraishi; T. Nakagome; F. Izumi; Y. Ishii; F. Nishikawa; T. Konishi (1997). "X-ray absorption fine structure and neutron diffraction analyses of de-intercalation behavior in the LiCoO2 and LiNiO2 systems". Journal of Power Sources. 68 (2): 536–539. Bibcode:1997JPS....68..536N. doi:10.1016/S0378-7753(97)02598-6.
- ^ a b Shao-Horn, Yang; Croguennec, Laurence; Delmas, Claude; Nelson, E. Chris; O'Keefe, Michael A. (July 2003). "Atomic resolution of lithium ions in LiCoO
2". Nature Materials. 2 (7): 464–467. doi:10.1038/nmat922. PMID 12806387. S2CID 34357573. - ^ a b H. J. Orman & P. J. Wiseman (January 1984). "Cobalt(III) lithium oxide, CoLiO
2: structure refinement by powder neutron diffraction". Acta Crystallographica Section C. 40 (1): 12–14. doi:10.1107/S0108270184002833. - ^ Qi, Zhaoxiang; Koenig, Gary M. (2016-08-16). "High-Performance LiCoO2Sub-Micrometer Materials from Scalable Microparticle Template Processing". ChemistrySelect. 1 (13): 3992–3999. doi:10.1002/slct.201600872. ISSN 2365-6549.
- ^ a b Qi, Zhaoxiang (August 2016). "High-Performance LiCoO2 Sub-Micrometer Materials from Scalable Microparticle Template Processing". ChemistrySelect. 1 (13): 3992–3999. doi:10.1002/slct.201600872.
- ^ a b Tang, W.; Liu, L. L.; Tian, S.; Li, L.; Yue, Y. B.; Wu, Y. P.; Guan, S. Y.; Zhu, K. (2010-11-01). "Nano-LiCoO2 as cathode material of large capacity and high rate capability for aqueous rechargeable lithium batteries". Electrochemistry Communications. 12 (11): 1524–1526. doi:10.1016/j.elecom.2010.08.024.
- ^ K. Mizushima, P. C. Jones, P. J. Wiseman, J. B. Goodenough (1980), "Li
xCoO
2 (0<x<1): A New Cathode Material for Batteries of High Energy Density". Materials Research Bulletin, volume 15, pages 783–789. doi:10.1016/0025-5408(80)90012-4 - ^ Oswald, Stefan; Gasteiger, Hubert A. (2023-03-01). "The Structural Stability Limit of Layered Lithium Transition Metal Oxides Due to Oxygen Release at High State of Charge and Its Dependence on the Nickel Content". Journal of The Electrochemical Society. 170 (3): 030506. doi:10.1149/1945-7111/acbf80. ISSN 0013-4651.
- ^ Doughty, Daniel; Pesaran, Ahmad. "Vehicle Battery Safety Roadmap Guidance" (PDF). National Renewable Energy Laboratory. Retrieved 19 January 2013.

