Nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group (−NO2) into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters (−ONO2) between alcohols and nitric acid (as occurs in the synthesis of nitroglycerin). The difference between the resulting molecular structures of nitro compounds and nitrates (NO−3) is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom (typically carbon or another nitrogen atom), whereas in nitrate esters (also called organic nitrates), the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom (nitrito group).
There are many major industrial applications of nitration in the strict sense; the most important by volume are for the production of nitroaromatic compounds such as nitrobenzene. The technology is long-standing and mature.[1][2][3]
Nitration reactions are notably used for the production of explosives, for example the conversion of guanidine to nitroguanidine and the conversion of toluene to trinitrotoluene (TNT). Nitrations are, however, of wide importance virtually all aromatic amines (anilines) are produced from nitro precursors. Millions of tons of nitroaromatics are produced annually.[2]
Aromatic nitration
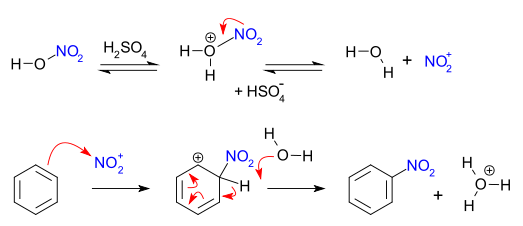
[edit]Typical nitrations of aromatic compounds rely on a reagent called "mixed acid", a mixture of concentrated nitric acid and sulfuric acids.[4][2] This mixture produces the nitronium ion (NO2+), which is the active species in aromatic nitration. This active ingredient, which can be isolated in the case of nitronium tetrafluoroborate,[5] also effects nitration without the need for the mixed acid. In mixed-acid syntheses sulfuric acid is not consumed and hence acts as a catalyst as well as an absorbent for water. In the case of nitration of benzene, the reaction is conducted at a warm temperature, not exceeding 50 °C. [6] The process is one example of electrophilic aromatic substitution, which involves the attack by the electron-rich benzene ring:
Alternative mechanisms have also been proposed, including one involving single electron transfer (SET).[7][8]
Scope
[edit]Selectivity can be a challenge. Often alternative products act as contaminants or are simply wasted. Considerable attention thus is paid to optimization of the reaction conditions. For example, the mixed acid can be derived from phosphoric or perchloric acids in place of sulfuric acid.[2]
Regioselectivity is strongly affected by substituents on aromatic rings (see electrophilic aromatic substitution). For example, nitration of nitrobenzene gives all three isomers of dinitrobenzenes in a ratio of 93:6:1 (respectively meta, ortho, para).[9] Electron-withdrawing groups such as other nitro are deactivating. Nitration is accelerated by the presence of activating groups such as amino, hydroxy and methyl groups also amides and ethers resulting in para and ortho isomers. In addition to regioselectivity, the degree of nitration is of interest. Fluorenone, for example, can be selectively trinitrated[10] or tetranitrated.[11]
The direct nitration of aniline with nitric acid and sulfuric acid, according to one source,[12] results in a 50/50 mixture of para- and meta-nitroaniline isomers. In this reaction the fast-reacting and activating aniline (ArNH2) exists in equilibrium with the more abundant but less reactive (deactivated) anilinium ion (ArNH3+), which may explain this reaction product distribution. According to another source,[13] a more controlled nitration of aniline starts with the formation of acetanilide by reaction with acetic anhydride followed by the actual nitration. Because the amide is a regular activating group the products formed are the para and ortho isomers. Heating the reaction mixture is sufficient to hydrolyze the amide back to the nitrated aniline.
Alternatives to nitric acid
[edit]Mixture of nitric and acetic acids or nitric acid and acetic anhydride is commercially important in the production of RDX, as amines are destructed by sulfuric acid. Acetyl nitrate had also been used as a nitration agent.[14] [15]
In the Wolffenstein–Böters reaction, benzene reacts with nitric acid and mercury(II) nitrate to give picric acid.
In the second half of the 20th century, new reagents were developed for laboratory usage, mainly N-nitro heterocyclic compounds.[16]
Ipso nitration
[edit]With aryl chlorides, triflates and nonaflates, ipso nitration may also take place.[17] The phrase ipso nitration was first used by Perrin and Skinner in 1971, in an investigation into chloroanisole nitration.[18] In one protocol, 4-chloro-n-butylbenzene is reacted with sodium nitrite in t-butanol in the presence of 0.5 mol% Pd2(dba)3, a biarylphosphine ligand and a phase-transfer catalyst to provide 4-nitro-n-butylbenzene.[19]
See also
[edit]References
[edit]- ^ *Schofield, K. (1971). Nitration and Aromatic Reactivity. Cambridge: Cambridge University Press.
- ^ a b c d Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- ^ Olahfirst1=G.A.; Malhotra, R.; Narang, S.C. (1989). Nitration: Methods and Mechanisms. NY: VCH. ISBN 978-0-471-18695-3.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ John McMurry Organic Chemistry 2nd Ed.
- ^ George A. Olah and Stephen J. Kuhn. "Benzonitrile, 2-methyl-3,5-dinitro-". Organic Syntheses; Collected Volumes, vol. 5, p. 480.
- ^ "Nitration of benzene and methylbenzene".
- ^ Esteves, P. M.; Carneiro, J. W. M.; Cardoso, S. P.; Barbosa, A. G. H.; Laali, K. K.; Rasul, G.; Prakash, G. K. S.; e Olah, G. A. (2003). "Unified Mechanism Concept of Electrophilic Aromatic Nitration Revisited: Convergence of Computational Results and Experimental Data". J. Am. Chem. Soc. 125 (16): 4836–49. doi:10.1021/ja021307w. PMID 12696903.
- ^ Queiroz, J. F.; Carneiro, J. W. M.; Sabino A. A.; Sparapan, R.; Eberlin, M. N.; Esteves, P. M. (2006). "Electrophilic Aromatic Nitration: Understanding Its Mechanism and Substituent Effects". J. Org. Chem. 71 (16): 6192–203. doi:10.1021/jo0609475. PMID 16872205.
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 665, ISBN 978-0-471-72091-1
- ^ E. O. Woolfolk and Milton Orchin. "2,4,7-Trinitrofluorenone". Organic Syntheses; Collected Volumes, vol. 3, p. 837.
- ^ Melvin S. Newman and H. Boden. "2,4,5,7-Tetranitrofluorenone". Organic Syntheses; Collected Volumes, vol. 5, p. 1029.
- ^ Web resource: warren-wilson.edu Archived 2012-03-20 at the Wayback Machine
- ^ Mechanism and synthesis Peter Taylor, Royal Society of Chemistry (Great Britain), Open University
- ^ Louw, Robert "Acetyl nitrate" e-EROS Encyclopedia of Reagents for Organic Synthesis 2001, 1-2. doi:10.1002/047084289X.ra032
- ^ Smith, Keith; Musson, Adam; Deboos, Gareth A. (1998). "A Novel Method for the Nitration of Simple Aromatic Compounds". The Journal of Organic Chemistry. 63 (23): 8448–8454. doi:10.1021/jo981557o.
- ^ Yang, Tao; Li, Xiaoqian; Deng, Shuang; Qi, Xiaotian; Cong, Hengjiang; Cheng, Hong-Gang; Shi, Liangwei; Zhou, Qianghui; Zhuang, Lin (2022-09-26). "From N–H Nitration to Controllable Aromatic Mononitration and Dinitration─The Discovery of a Versatile and Powerful N -Nitropyrazole Nitrating Reagent". JACS Au. 2 (9): 2152–2161. doi:10.1021/jacsau.2c00413. ISSN 2691-3704. PMC 9516713. PMID 36186553.
- ^ Prakash, G.; Mathew, T. (2010). "Ipso-Nitration of Arenes". Angewandte Chemie International Edition in English. 49 (10): 1726–1728. doi:10.1002/anie.200906940. PMID 20146295.
- ^ Perrin, C. L.; Skinner, G. A. (1971). "Directive effects in electrophilic aromatic substitution ("ipso factors"). Nitration of haloanisoles". Journal of the American Chemical Society. 93 (14): 3389. doi:10.1021/ja00743a015.
- ^ Fors, B.; Buchwald, S. (2009). "Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics". Journal of the American Chemical Society. 131 (36): 12898–12899. doi:10.1021/ja905768k. PMC 2773681. PMID 19737014.