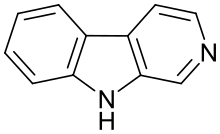
Β-Carboline
This article needs additional citations for verification. (December 2007) |
| |

| |
| Names | |
|---|---|
| IUPAC name
9H-β-carboline
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.418 |
| MeSH | norharman |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H8N2 | |
| Molar mass | 168.20 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Carboline (9H-pyrido[3,4-b]indole), also known as norharmane, is a nitrogen containing heterocycle. It is also the prototype of a class of indole alkaloid compounds known as β-carbolines.[1]
Pharmacology
β-Carboline alkaloids are widespread in plants and animals, and frequently act as GABAA inverse agonists. As components of the liana Banisteriopsis caapi, the β-carbolines harmine, harmaline, and tetrahydroharmine play a pivotal role in the pharmacology of the indigenous psychedelic drug ayahuasca by preventing the breakdown of dimethyltryptamine in the gut by reversibly inhibiting monoamine oxidase, thus making it psychoactive upon oral administration. Some β-carbolines, notably tryptoline and pinoline, may be formed naturally in the human body. The latter is possibly implicated along with melatonin in the role of the pineal gland in regulating the sleep-wake cycle.[citation needed] β-carboline is a GABAA benzodiazepine site inverse agonist and can therefore have convulsive, anxiogenic and memory enhancing effects.[2] 3-hydroxymethyl-beta-carboline blocks the sleep-promoting effect of flurazepam in rodents, and by itself can decrease sleep in a dose-dependent manner.[3] 9-Methyl-β-carbolines appear to induce DNA damage when exposed to ultra-violet light.[4]
Structure
β-Carboline belongs to the group of indole alkaloids and consist of pyridine ring that is fused to an indole skeleton.[5] The structure of β-carboline is similar to that of tryptamine, with the ethylamine chain re-connected to the indole ring via an extra carbon atom, to produce a three-ringed structure. The biosynthesis of β-carbolines is believed to follow this route from analogous tryptamines.[6] Different levels of saturation are possible in the third ring, which is indicated here in the structural formula by colouring the optionally double bonds red and blue:
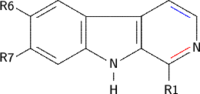
Examples of β-carbolines
Some of the more important β-carbolines are tabulated by structure below. Their structures may contain the aforementioned bonds marked by red or blue.
| Short Name | Red Bond | Blue Bond | R1 | R6 | R7 | R9 | Structure |
|---|---|---|---|---|---|---|---|
| β-Carboline | × | × | H | H | H | H | 
|
| Tryptoline | H | H | H | H | 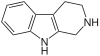
| ||
| Pinoline | H | OCH3 | H | H | 
| ||
| Harmane | × | × | CH3 | H | H | H | 
|
| Harmine | × | × | CH3 | H | OCH3 | H | 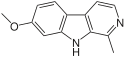
|
| Harmaline | × | CH3 | H | OCH3 | H | 
| |
| Tetrahydroharmine | CH3 | H | OCH3 | H | 
| ||
| 9-Methyl-β-carboline | × | × | H | H | H | CH3 | 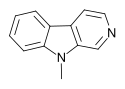
|
Occurrence in nature

Eight plant families are known to express 64 different kinds of β-carboline alkaloids. By dry weight, the seeds of Peganum harmala (Syrian Rue) contain between 0.16%[7] and 5.9%[8] β-carboline alkaloids.
As a result of the presence of β-carbolines in the cuticle of scorpions, their skin is known to fluoresce when exposed to certain wavelengths of ultraviolet light such as that produced by a blacklight.[9]
A group of β-carboline derivatives, termed eudistomins were extracted from ascidians (marine tunicates of the family Ascidiacea), like Ritterella sigillinoides,[10] Lissoclinum fragile [11] or Pseudodistoma aureum.[12] Nostocarboline was isolated from freshwater cyanobacterium.
β-carboline derivatives are known to induce the production of secondary metabolite in various soil dwelling “Streptomyces” species.[13][14] Defined as biomediator, these molecules are known to enhance the expression of secondary metabolite biosynthetic genes by binding to Large ATP-binding regulator of the LuxR family.
See also
References
- ^ "β-Carboline Alkaloid Constituents from a Thermoactinomyces SP. Strain Isolated from Livingston Island, Antarctica". doi:10.5504/BBEQ.2012.0021.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Venault P, Chapouthier G (2007). "From the behavioral pharmacology of beta-carbolines to seizures, anxiety, and memory". ScientificWorldJournal. 7: 204–23. doi:10.1100/tsw.2007.48. PMC 5901106. PMID 17334612.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mendelson, W.; Cain, M; Cook, J.; Paul, S.; Skolnick, P (1983-01-28). "A benzodiazepine receptor antagonist decreases sleep and reverses the hypnotic actions of flurazepam". Science. 219 (4583): 414–416. Bibcode:1983Sci...219..414M. doi:10.1126/science.6294835. ISSN 0036-8075. PMID 6294835. S2CID 43038332.
- ^ Vignoni M, Rasse-Suriani FA, Butzbach K, Erra-Balsells R, Epe B, Cabrerizo FM (2013). "Mechanisms of DNA damage by photoexcited 9-methyl-β-carbolines". Org Biomol Chem. 11 (32): 5300–9. doi:10.1039/c3ob40344k. PMID 23842892.
- ^ The Encyclopedia of Psychoactive Plants: Ethnopharmacology and its Applications. Ratsch, Christian. Park Street Press c. 2005
- ^ Baiget, Jessica; Llona-Minguez, Sabin; Lang, Stuart; MacKay, Simon P; Suckling, Colin J; Sutcliffe, Oliver B (2011). "Manganese dioxide mediated one-pot synthesis of methyl 9H-pyrido[3,4-b]indole-1-carboxylate: Concise synthesis of alangiobussinine". Beilstein Journal of Organic Chemistry. 7: 1407–11. doi:10.3762/bjoc.7.164. PMC 3201054. PMID 22043251.
- ^ Hemmateenejad B, Abbaspour A, Maghami H, Miri R, Panjehshahin MR (Aug 2006). "Partial least squares-based multivariate spectral calibration method for simultaneous determination of beta-carboline derivatives in Peganum harmala seed extracts". Anal Chim Acta. 575 (2): 290–9. doi:10.1016/j.aca.2006.05.093. PMID 17723604.
- ^ Herraiz T, González D, Ancín-Azpilicueta C, Arán VJ, Guillén H (2010). "beta-Carboline alkaloids in Peganum harmala and inhibition of human monoamine oxidase (MAO)". Food Chem. Toxicol. 48 (3): 839–43. doi:10.1016/j.fct.2009.12.019. hdl:10261/77694. PMID 20036304.
- ^ Stachel SJ, Stockwell SA, van Vranken DL (August 1999). "The fluorescence of scorpions and cataractogenesis". Chem. Biol. 6 (8): 531–9. doi:10.1016/S1074-5521(99)80085-4. PMID 10421760.
- ^ Lake RJ, Blunt JW, Munro MH (1989). "Eudistomins from the New Zealand ascidian Ritterella sigillinoides". Aust. J. Chem. 42 (7): 1201–1206. doi:10.1071/CH9891201.
- ^ Badre A, Boulanger A, Abou-Mansour E, Banaigs B, Combaut G, Francisco C (April 1994). "Eudistomin U and isoeudistomin U, new alkaloids from the Caribbean ascidian Lissoclinum fragile". J. Nat. Prod. 57 (4): 528–533. doi:10.1021/np50106a016. PMID 8021654.
- ^ Davis RA, Carroll AR, Quinn RJ (June 1998). "Eudistomin V, a new β-carboline from the Australian ascidian Pseudodistoma aureum". J. Nat. Prod. 61 (7): 959–960. doi:10.1021/np9800452. PMID 9677285.
- ^ Panthee, S; Takahashi, S; Hayashi, T; Shimizu, T; Osada, H (9 April 2019). "β-carboline biomediators induce reveromycin production in Streptomyces sp. SN-593". Scientific Reports. 9 (1): 5802. doi:10.1038/s41598-019-42268-w. PMID 30967594.
- ^ Panthee, S; Kito, N; Hayashi, T; Shimizu, T; Ishikawa, J; Hamamoto, H; Osada, H; Takahashi, S (23 June 2020). "β-carboline chemical signals induce reveromycin production through a LuxR family regulator in Streptomyces sp. SN-593". Scientific Reports. 10 (1): 10230. doi:10.1038/s41598-020-66974-y. PMID 32576869.
External links
- Beta-Carbolines at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- TiHKAL #44
- TiHKAL in general
- Beta-carbolines in coffee
- Farzin D, Mansouri N (July 2006). "Antidepressant-like effect of harmane and other beta-carbolines in the mouse forced swim test". Eur Neuropsychopharmacol. 16 (5): 324–8. doi:10.1016/j.euroneuro.2005.08.005. PMID 16183262.
