Abacavir: Difference between revisions
Reverted good faith edits by 98.159.203.25 (talk): ? (TW) |
Reorganized the introductory paragraphs and added citations where needed. Briefly mentioned the mechanism of abacavir, removed the portion about cost as it is variable, expanded upon adverse effects, and briefly mentioned formulations. |
||
| Line 55: | Line 55: | ||
| StdInChIKey = MCGSCOLBFJQGHM-SCZZXKLOSA-N |
| StdInChIKey = MCGSCOLBFJQGHM-SCZZXKLOSA-N |
||
}} |
}} |
||
'''Abacavir''', commonly abbreviated “'''ABC''',” is an antiretroviral medication used to treat [[HIV/AIDS|HIV infection]].<ref name=":0">{{Cite journal|last=Yuen|first=Geoffrey J.|last2=Weller|first2=Steve|last3=Pakes|first3=Gary E.|date=2012-09-13|title=A Review of the Pharmacokinetics of Abacavir|url=http://link.springer.com/article/10.2165/00003088-200847060-00001|journal=Clinical Pharmacokinetics|language=en|volume=47|issue=6|pages=351–371|doi=10.2165/00003088-200847060-00001|issn=0312-5963}}</ref><ref>{{Cite web|url=https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/292/drug-name-abbreviations|title=Drug Name Abbreviations {{!}} Adult and Adolescent ARV Guidelines {{!}} AIDSinfo|website=AIDSinfo|access-date=2016-11-08}}</ref> It belongs to the [[Reverse-transcriptase inhibitor|nucleoside reverse transcriptase inhibitor]] (NRTI) class of antiretroviral medications, which work by inhibiting reverse transcriptase, a key enzyme for viral multiplication, ultimately preventing duplication of the HIV virus.<ref name=":1">{{Cite web|url=http://www.hiv.va.gov/patient/treat/NRTIs.asp|title=Nucleoside reverse transcriptase inhibitors (NRTIs or 'nukes') - HIV/AIDS|website=www.hiv.va.gov|access-date=2016-11-08}}</ref> Within the NRTI class, abacavir is a [[carbocyclic nucleoside]]. Similar to other NRTI’s, abacavir is used in combination with other HIV medications, and is not indicated for use as monotherapy in the treatment of HIV.<ref>{{Cite web|url=https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv-guidelines/12/what-not-to-use|title=What Not to Use {{!}} Adult and Adolescent ARV Guidelines {{!}} AIDSinfo|website=AIDSinfo|access-date=2016-11-08}}</ref> Abacavir is on the [[WHO Model List of Essential Medicines|World Health Organization’s List of Essential Medicines]], which is updated every 2 years and includes a “list of minimum medicine needs for a basic health-care system."<ref>{{Cite web|url=http://www.who.int/medicines/publications/essentialmedicines/en/|title=WHO Model Lists of Essential Medicines|website=World Health Organization|language=en-GB|access-date=2016-11-08}}</ref> |
|||
<!-- Definition and medical uses --> |
|||
'''Abacavir''' ('''ABC''') is an [[antiretroviral medication]] used to prevent and treat [[HIV/AIDS]].<ref name=AHFS2015>{{cite web|title=Abacavir Sulfate|url=http://www.drugs.com/monograph/abacavir-sulfate.html|publisher=The American Society of Health-System Pharmacists|accessdate=31 July 2015}}</ref> Viral strains that are resistant to [[zidovudine]] (AZT) or [[lamivudine]] (3TC) are generally, but not always, sensitive to abacavir. |
|||
Abacavir is generally well tolerated; however, it has [[Boxed warning|black box warnings]] for hypersensitivity, liver damage, and lactic acidosis.<ref name=":0" /> Genetic testing can indicate whether an individual is likely to exhibit hypersensitivity.<ref name=":0" /> Common symptoms of abacavir-associated hypersensitivity include rash, nausea, vomiting, and fatigue.<ref name=":0" /> |
|||
<!-- Side effects and mechanism--> |
|||
It is well tolerated: the main side effect is [[hypersensitivity]], which can be severe, and in rare cases, fatal. Genetic testing can indicate whether an individual is likely to be hypersensitive; over 90% of people can safely take abacavir. It is of the [[nucleoside]] analog [[reverse transcriptase inhibitor]] (NRTI) type and is in the class of [[carbocyclic nucleoside]]s. |
|||
Commonly, abacavir is formulated in combination with other HIV medications, such as [[abacavir/lamivudine/zidovudine]], [[abacavir/dolutegravir/lamivudine]], and [[abacavir/lamivudine]].<ref name=":0" /><ref name=":1" /> It also comes individually formulated as a tablet or a solution.<ref name=":0" /> |
|||
<!-- Society and culture --> |
|||
It is on the [[World Health Organization's List of Essential Medicines]], the most important medication needed in a basic [[health system]].<ref>{{cite web|title=WHO Model List of EssentialMedicines|url=http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1|work=World Health Organization|accessdate=22 April 2014|date=October 2013}}</ref> It is available in the combination formulations [[abacavir/lamivudine/zidovudine]], [[abacavir/dolutegravir/lamivudine]], and [[abacavir/lamivudine]]. As of 2015 the cost for a typical month of medication in the United States is more than 200 USD.<ref name=Ric2015>{{cite book|last1=Hamilton|first1=Richart|title=Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition|date=2015|publisher=Jones & Bartlett Learning|isbn=9781284057560|page=64}}</ref> |
|||
== Medical uses == |
== Medical uses == |
||
Revision as of 17:39, 8 November 2016
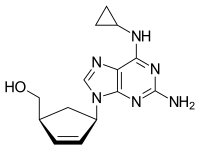 | |
 Chemical structure of abacavir | |
| Clinical data | |
|---|---|
| Pronunciation | /ʌ.bæk.ʌ.vɪər/ |
| Trade names | Ziagen |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699012 |
| Pregnancy category |
|
| Routes of administration | Oral (solution or tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 83% |
| Metabolism | Hepatic |
| Elimination half-life | 1.54 ± 0.63 h |
| Excretion | Renal (1.2% abacavir, 30% 5'-carboxylic acid metabolite, 36% 5'-glucuronide metabolite, 15% unidentified minor metabolites). Fecal (16%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.149.341 |
| Chemical and physical data | |
| Formula | C14H18N6O |
| Molar mass | 286.332 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 165 °C (329 °F) |
| |
| |
| (verify) | |
Abacavir, commonly abbreviated “ABC,” is an antiretroviral medication used to treat HIV infection.[2][3] It belongs to the nucleoside reverse transcriptase inhibitor (NRTI) class of antiretroviral medications, which work by inhibiting reverse transcriptase, a key enzyme for viral multiplication, ultimately preventing duplication of the HIV virus.[4] Within the NRTI class, abacavir is a carbocyclic nucleoside. Similar to other NRTI’s, abacavir is used in combination with other HIV medications, and is not indicated for use as monotherapy in the treatment of HIV.[5] Abacavir is on the World Health Organization’s List of Essential Medicines, which is updated every 2 years and includes a “list of minimum medicine needs for a basic health-care system."[6]
Abacavir is generally well tolerated; however, it has black box warnings for hypersensitivity, liver damage, and lactic acidosis.[2] Genetic testing can indicate whether an individual is likely to exhibit hypersensitivity.[2] Common symptoms of abacavir-associated hypersensitivity include rash, nausea, vomiting, and fatigue.[2]
Commonly, abacavir is formulated in combination with other HIV medications, such as abacavir/lamivudine/zidovudine, abacavir/dolutegravir/lamivudine, and abacavir/lamivudine.[2][4] It also comes individually formulated as a tablet or a solution.[2]
Medical uses
Abacavir tablets and oral solution, in combination with other antiretroviral agents, are indicated for the treatment of HIV-1 infection.
Abacavir should always be used in combination with other antiretroviral agents. Abacavir should not be added as a single agent when antiretroviral regimens are changed due to loss of virologic response.
Side effects
Common reactions include nausea, headache, fatigue, vomiting, hypersensitivity reaction, diarrhea, fever/chills, depression, rash, anxiety, URI, ALT, AST elevated, hypertriglyceridemia, and lipodystrophy.[7]
Patients with liver disease should be cautious about using abacavir because of the possibility that it can aggravate the condition. The use of nucleoside drugs such as abacavir can very rarely cause lactic acidosis. Resistance to abacavir has developed in laboratory versions of HIV which are also resistant to other HIV-specific antiretrovirals such as lamivudine, didanosine and zalcitabine. HIV strains that are resistant to protease inhibitors are not likely to be resistant to abacavir.
Abacavir is contraindicated for use in infants under 3 months of age.
Little is known about the effects of Abacavir overdose. Overdose victims should be taken to a hospital emergency room for treatment.
Hypersensitivity syndrome
Hypersensitivity to abacavir is strongly associated with a specific allele at the human leukocyte antigen B locus namely HLA-B*57:01.[8][9] There is an association between the prevalence of HLA-B*5701 and ancestry. The prevalence of the allele is estimated to be 3.4 to 5.8% on average in populations of European ancestry, 17.6% in Indian Americans, 3.0% in Hispanic Americans, and 1.2% in Chinese Americans.[10][11] There is significant variability in the prevalence of HLA-B*5701 among African populations. In African Americans, the prevalence is estimated to be 1.0% on average, 0% in the Yoruba from Nigeria, 3.3% in the Luhya from Kenya, and 13.6% in the Masai from Kenya, although the average values are derived from highly variable frequencies within sample groups.[12]
Common symptoms of abacavir hypersensitivity syndrome include fever, malaise, nausea, and diarrhea; some patients may also develop a skin rash.[13] Symptoms of AHS typically manifest within six weeks of treatment using abacavir, although they may be confused with symptoms of HIV, immune restoration disease, hypersensitivity syndromes associated with other drugs, or infection.[14] The FDA released an alert concerning abacavir and abacavir-containing medications on July 24, 2008,[15] and the FDA-approved drug label for abacavir recommends pre-therapy screening for the HLA-B*5701 allele, and the use of alternative therapy in subjects with this allele.[16] Additionally, both the Clinical Pharmacogenetics Implementation Consortium (CPIC) and Dutch Pharmacogenetics Working Group (DPWG) recommend use of an alternative therapy in individuals with the HLA-B*5701 allele.[17][18]
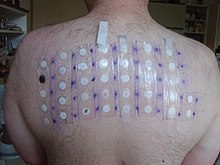
Skin-patch testing may also be used to determine whether an individual will experience a hypersensitivity reaction to abacavir, although some patients susceptible to developing AHS may not react to the patch test.[19]
The development of suspected hypersensitivity reactions to abacavir requires immediate and permanent discontinuation of abacavir therapy in all patients, including patients who do not possess the HLA-B*5701 allele. On March 1, 2011, the FDA informed the public about an ongoing safety review of abacavir and a possible increased risk of heart attack associated with the drug. However, a meta-analysis of 26 studies conducted by the FDA did not find any association between abacavir use and heart attack [20][21]
Immunopathogenesis
The mechanism underlying abacavir hypersensitivity syndrome is related to the change in the HLA-B*5701 protein product. Abacavir binds with high specificity to the HLA-B*5701 protein, changing the shape and chemistry of the antigen-binding cleft. This results in a change in immunological tolerance and the subsequent activation of abacavir-specific cytotoxic T cells, which produce a systemic reaction known as abacavir hypersensitivity syndrome.[22]
Mechanism of action
ABC a chiral purine carbocyclic nucleoside, is a prodrug of the guanosine analog (-) carbovir.[23] Its target is the viral reverse transcriptase enzyme. ABC is converted intracellularly to the triphosphate of (-)carbovir which competes with guanosine triphosphate for incorporation into the DNA strand. When reverse transcriptase incorporates the ABC product (-)carbovir triphosphate, transcription is terminated because there is no 3' hydroxyl group on the molecule to make a phosphodiester bond to the next nucleotide in the DNA sequence.[24]
Pharmacokinetics
Abacavir is given orally and has a high bioavailability (83%). It is metabolised primarily through alcohol dehydrogenase or glucuronyl transferase. It is capable of crossing the blood–brain barrier.
History
Abacavir was approved by the Food and Drug Administration (FDA) on December 18, 1998 and is thus the fifteenth approved antiretroviral drug in the United States. Its patent expired in the United States on 2009-12-26.
Robert Vince and Susan Daluge in the '80s along with a visiting scientist from China, Mei Hua, developed the medication.[25][26][23]
Synthesis

References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b c d e f Yuen, Geoffrey J.; Weller, Steve; Pakes, Gary E. (2012-09-13). "A Review of the Pharmacokinetics of Abacavir". Clinical Pharmacokinetics. 47 (6): 351–371. doi:10.2165/00003088-200847060-00001. ISSN 0312-5963.
- ^ "Drug Name Abbreviations | Adult and Adolescent ARV Guidelines | AIDSinfo". AIDSinfo. Retrieved 2016-11-08.
- ^ a b "Nucleoside reverse transcriptase inhibitors (NRTIs or 'nukes') - HIV/AIDS". www.hiv.va.gov. Retrieved 2016-11-08.
- ^ "What Not to Use | Adult and Adolescent ARV Guidelines | AIDSinfo". AIDSinfo. Retrieved 2016-11-08.
- ^ "WHO Model Lists of Essential Medicines". World Health Organization. Retrieved 2016-11-08.
- ^ https://online.epocrates.com/noFrame/showPage.do?method=drugs&MonographId=2043&ActiveSectionId=5
- ^ Mallal, S., Phillips, E., Carosi, G.; et al. (2008). "HLA-B*5701 screening for hypersensitivity to abacavir". New England Journal of Medicine. 358 (6): 568–579. doi:10.1056/nejmoa0706135. PMID 18256392.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rauch, A., Nolan, D., Martin, A.; et al. (2006). "Prospective genetic screening decreases the incidence of abacavir hypersensitivity reactions in the Western Australian HIV cohort study". Clinical Infectious Diseases. 43 (1): 99–102. doi:10.1086/504874. PMID 16758424.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Heatherington; et al. (2002). "Genetic variations in HLA-B region and hypersensitivity reactions to abacavir". Lancet. 359 (9312): 1121–1122. doi:10.1016/s0140-6736(02)08158-8. PMID 11943262.
- ^ Mallal; et al. (2002). "Association between presence of HLA*B5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir". Lancet. 359 (9308): 727–732. doi:10.1016/s0140-6736(02)07873-x. PMID 11888582.
- ^ Rotimi, C.N.; Jorde, L.B. (2010). "Ancestry and disease in the age of genomic medicine". New England Journal of Medicine. 363 (16): 1551–1558. doi:10.1056/nejmra0911564. PMID 20942671.
- ^ Phillips, E., Mallal, S. (2009). "Successful translation of pharmacogenetics into the clinic". Molecular Diagnosis & Therapy. 13: 1–9. doi:10.1007/bf03256308.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Phillips, E., Mallal S. (2007). "Drug hypersensitivity in HIV". Current Opinion in Allergy and Clinical Immunology. 7 (4): 324–330. doi:10.1097/aci.0b013e32825ea68a. PMID 17620824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm123927.htm Accessed November 29, 2013.
- ^ http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ca73b519-015a-436d-aa3c-af53492825a1
- ^ Swen JJ, Nijenhuis M, de Boer A, et al. (May 2011). "Pharmacogenetics: from bench to byte--an update of guidelines". Clin Pharmacol Ther. 89 (5): 662–73. doi:10.1038/clpt.2011.34. PMID 21412232.
- ^ Martin MA, Hoffman JM, Freimuth RR, et al. (May 2014). "Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update". Clin Pharmacol Ther. 95 (5): 499–500. doi:10.1038/clpt.2014.38. PMC 3994233. PMID 24561393.
- ^ Shear, N.H., Milpied, B., Bruynzeel, D.P.; et al. (2008). "A review of drug patch testing and implications for HIV clinicians". AIDS. 22 (9): 999–1007. doi:10.1097/qad.0b013e3282f7cb60. PMID 18520343.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ http://www.drugs.com/fda/abacavir-ongoing-safety-review-possible-increased-risk-heart-attack-12914.html Accessed November 29, 2013.
- ^ Ding X, Andraca-Carrera E, Cooper C, et al. (December 2012). "No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis". J Acquir Immune Defic Syndr. 61 (4): 441–7. doi:10.1097/QAI.0b013e31826f993c. PMID 22932321.
- ^ Illing, PT; et al. (2012). "Immune self-reactivity triggered by drug-modified HLA-peptide repertoire". Nature. doi:10.1038/nature11147.
- ^ a b Daluge SM, Good SS, Faletto MB, Miller WH, St Clair MH, Boone LR, Tisdale M, Parry NR, Reardon JE, Dornsife RE, Averett DR (May 1997). "1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity". Antimicrobial agents and chemotherapy. 41 (5): 1082–1093. PMC 163855. PMID 9145874.
- ^ The Pharmacological Basics of Therapeutics, 12th Ed. Goodman and Gilman
- ^ "Dr. Robert Vince - 2010 Inductee". Minnesota Inventors Hall of Fame. Minnesota Inventors Hall of Fame. Retrieved 10 February 2016.
- ^ Vince, R. University of Minnesota. University of Minnesota http://drugdesign.umn.edu/bio/cdd-faculty-staff/robert-vince.
{{cite web}}: Missing or empty|title=(help) - ^ Crimmins, M. T.; King, B. W. (1996). "An Efficient Asymmetric Approach to Carbocyclic Nucleosides: Asymmetric Synthesis of 1592U89, a Potent Inhibitor of HIV Reverse Transcriptase". The Journal of Organic Chemistry. 61 (13): 4192–4193. doi:10.1021/jo960708p. PMID 11667311.
