Vitamin D: Difference between revisions
→Weight loss: copyedit and add content with review using Citation bot |
→Research: copyedit per Hoel review; add content with refs on obesity associations assisted by Citation bot; replace weak Hacker ref with NIH sources edited with ProveIt |
||
| Line 426: | Line 426: | ||
Vitamin D content in typical foods is reduced variably by cooking, such as by boiling, [[frying]] or baking.<ref name="fc">{{cite journal|last1=Jakobsen|first1=Jette|last2=Knuthsen|first2=Pia|title=Stability of vitamin D in foodstuffs during cooking|journal=Food Chemistry|volume=148|pages=170–175|doi=10.1016/j.foodchem.2013.10.043|pmid=24262542|year=2014}}</ref> Boiled, fried and baked foods retained 69–89% of original vitamin D.<ref name=fc/> |
Vitamin D content in typical foods is reduced variably by cooking, such as by boiling, [[frying]] or baking.<ref name="fc">{{cite journal|last1=Jakobsen|first1=Jette|last2=Knuthsen|first2=Pia|title=Stability of vitamin D in foodstuffs during cooking|journal=Food Chemistry|volume=148|pages=170–175|doi=10.1016/j.foodchem.2013.10.043|pmid=24262542|year=2014}}</ref> Boiled, fried and baked foods retained 69–89% of original vitamin D.<ref name=fc/> |
||
==Research== |
==Research and education== |
||
A |
A 2016 review stated that reports of favorable health outcomes deriving from moderate sun exposure and adequate vitamin D concentration or vitamin D supplementation were not adequately described in the medical literature.<ref name="hoel">{{cite journal|last1=Hoel|first1=David G.|last2=Berwick|first2=Marianne|last3=de Gruijl|first3=Frank R.|last4=Holick|first4=Michael F.|title=The risks and benefits of sun exposure 2016|journal=Dermato-Endocrinology |year=2016|volume=8|issue=1|pages=e1248325|doi=10.1080/19381980.2016.1248325|pmid=27942349| |
||
url=http://tandfonline.com/doi/full/10.1080/19381980.2016.1248325}}</ref> While medical authorities in the United States have recommended that men, women and children reduce their sun exposure based on concerns about skin cancer, the majority of Americans have vitamin D deficiency that may lead to increased risk of diseases possibly preventable by adequate sun exposure and the resulting higher vitamin D levels.<ref name=hoel/> The review proposed various potential health benefits from sun exposure and improved vitamin D status, including the potential for reduced risk of different types of cancer, cardiovascular diseases, [[cognitive disorder]]s, [[myopia]] and [[macular degeneration]], diabetes and [[multiple sclerosis]], all of which are being evaluated in clinical trials.<ref name=hoel/><ref>{{cite journal|journal=Am J Med Sci|year=2009|volume=338|issue=1|pages=34-9|doi=10.1097/MAJ.0b013e3181aaee78|title=Vitamin D and health in the 21st century: federal initiatives to advance research|author=Costello RB|pmid=19593101}}</ref><ref>{{Cite web |url=https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/vitamin-d-fact-sheet#q4 |title=How is vitamin D being studied now in clinical cancer research? |publisher=National Cancer Institute, US National Institutes of Health |location=Bethesda, MD |publication-date=21 October 2013}}</ref> The ongoing United States [[National Health and Nutrition Examination Survey]] (NHANES)<ref>{{Cite web |url=https://www.cdc.gov/nchs/nhanes/about_nhanes.htm |title=About the National Health and Nutrition Examination Survey |publisher=National Center for Health Statistics, US Centers for Disease Control |location=Atlanta, GA |publication-date=6 November 2015}}</ref> is assessing the prevalence of numerous diseases possibly associated with vitamin D deficiency.<ref>{{cite journal|journal=Nutr Res|year=2011|volume=31|issue=1|pages=48-54|doi=10.1016/j.nutres.2010.12.001|title=Prevalence and correlates of vitamin D deficiency in US adults|authors=Forrest KY, Stuhldreher WL|pmid=21310306}}</ref> Starting in 2014, the United States [[National Institutes of Health]] and Office of Dietary Supplements established a Vitamin D Initiative to track current research and provide education to consumers.<ref>{{Cite web |url=https://ods.od.nih.gov/Research/VitaminD.aspx |title=ODS Vitamin D Initiative |publisher=Office of Dietary Supplements, US National Institutes of Health |location=Bethesda, MD |publication-date=2014}}</ref> |
|||
European research is assessing vitamin D intake levels in association with disease incidence and policies for judicious sun exposure, dietary recommendations, food fortification, and vitamin D supplementation.<ref>{{cite journal|journal=Nutr Bull|year=2014|volume=39|issue=4|pages=322-350|title=Vitamin D: An overview of vitamin D status and intake in Europe|authors=Spiro A, Buttriss JL|pmid=25635171|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4288313/}}</ref> |
|||
There are a number of randomized controlled trials in progress as of 2015.<ref>Hacker, John. "[http://www.mdedge.com/jfponline/article/99947/practice-management/lets-talk-about-evidence Let's talk about the evidence]." ''J Fam Pract''. 2015 June;64(6):337.</ref> |
|||
Preliminary studies in overweight or obese [[soldier]]s<ref>{{cite journal|pmid=25643393|year=2015|author1=Funderburk|first1=L. K.|title=Vitamin D status among overweight and obese soldiers|journal=Military Medicine|volume=180|issue=2|pages=237–40|last2=Daigle|first2=K|last3=Arsenault|first3=J. E.|doi=10.7205/MILMED-D-14-00041}}</ref> and children<ref>{{cite journal|pmid=22072738|pmc=3251943|year=2012|author1=Olson|first1=M. L.|title=Vitamin D deficiency in obese children and its relationship to glucose homeostasis|journal=The Journal of Clinical Endocrinology & Metabolism|volume=97|issue=1|pages=279–85|last2=Maalouf|first2=N. M.|last3=Oden|first3=J. D.|last4=White|first4=P. C.|last5=Hutchison|first5=M. R.|doi=10.1210/jc.2011-1507}}</ref><ref>{{cite journal|pmid=23266927|year=2013|author1=Turer|first1=C. B.|title=Prevalence of vitamin D deficiency among overweight and obese US children|journal=Pediatrics|volume=131|issue=1|pages=e152–61|last2=Lin|first2=H|last3=Flores|first3=G|doi=10.1542/peds.2012-1711|url=http://pediatrics.aappublications.org/content/pediatrics/131/1/e152.full.pdf}}</ref><ref>{{cite journal|pmid=25315624|year=2015|author1=Pyrżak|first1=B|title=Metabolic and immunological consequences of vitamin D deficiency in obese children|journal=Advances in experimental medicine and biology|volume=840|last2=Witkowska-Sędek|first2=E|last3=Krajewska|first3=M|last4=Demkow|first4=U|last5=Kucharska|first5=A. M.|journal=Adv Exp Med Biol|year=2015|volume=840|page=13-19|doi=10.1007/5584_2014_81}}</ref> established an association that blood levels of vitamin D are proportionately suppressed by fat mass, indicating a need to develop specific clinical screening and treatment guidance for these populations. |
|||
==References== |
==References== |
||
Revision as of 18:44, 15 December 2016
| Vitamin D | |
|---|---|
| Drug class | |
 Cholecalciferol (D3) | |
| Class identifiers | |
| Use | Rickets, osteoporosis, vitamin D deficiency |
| ATC code | A11CC |
| Biological target | vitamin D receptor |
| Clinical data | |
| Drugs.com | MedFacts Natural Products |
| External links | |
| MeSH | D014807 |
| Legal status | |
| In Wikidata | |
Vitamin D refers to a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, iron, magnesium, phosphate, and zinc. In humans, the most important compounds in this group are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol).[1] Cholecalciferol and ergocalciferol can be ingested from the diet and from supplements.[1][2][3] Very few foods contain vitamin D; synthesis of vitamin D (specifically cholecalciferol) in the skin is the major natural source of the vitamin. Dermal synthesis of vitamin D from cholesterol is dependent on sun exposure (specifically UVB radiation).
Vitamin D from the diet or dermal synthesis from sunlight is biologically inactive; activation requires enzymatic conversion (hydroxylation) in the liver and kidney. Evidence indicates the synthesis of vitamin D from sun exposure is regulated by a negative feedback loop that prevents toxicity, but because of uncertainty about the cancer risk from sunlight, no recommendations are issued by the Institute of Medicine (US) for the amount of sun exposure required to meet vitamin D requirements. Accordingly, the Dietary Reference Intake for vitamin D assumes no synthesis occurs and all of a person's vitamin D is from food intake, although that will rarely occur in practice. As vitamin D is synthesized in adequate amounts by most mammals exposed to sunlight[citation needed], it is not strictly a vitamin, and may be considered a hormone as its synthesis and activity occur in different locations. Vitamin D has a significant role in calcium homeostasis and metabolism. Its discovery was due to effort to find the dietary substance lacking in rickets (the childhood form of osteomalacia).[4]
Beyond its use to prevent osteomalacia or rickets, the evidence for other health effects of vitamin D supplementation in the general population is inconsistent.[5][6] The effect of vitamin D supplementation on mortality is not clear, with one meta-analysis finding a decrease in mortality in elderly people,[7] and another concluding no clear justification exists for recommending vitamin D.[8]
In the liver, cholecalciferol (vitamin D3) is converted to calcifediol. Ergocalciferol (vitamin D2) is converted in the liver to 25-hydroxyergocalciferol (a.k.a. 25-hydroxyvitamin D2 — abbreviated 25(OH)D2). These two specific vitamin D metabolites are measured in serum to determine a person's vitamin D status.[9][10] Part of the calcifediol is converted by the kidneys to calcitriol, the biologically active form of vitamin D.[11] Calcitriol circulates as a hormone in the blood, regulating the concentration of calcium and phosphate in the bloodstream and promoting the healthy growth and remodeling of bone. Calcitriol also affects neuromuscular and immune function.[12]
Types
| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | Mixture of molecular compounds of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 
|
| Vitamin D3 | cholecalciferol (made from 7-dehydrocholesterol in the skin). | 
|
| Vitamin D4 | 22-dihydroergocalciferol | 
|
| Vitamin D5 | sitocalciferol (made from 7-dehydrositosterol) | 
|
Several forms (vitamers) of vitamin D exist. The two major forms are vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol; vitamin D without a subscript refers to either D2 or D3 or both. These are known collectively as calciferol.[13] Vitamin D2 was chemically characterized in 1931. In 1935, the chemical structure of vitamin D3 was established and proven to result from the ultraviolet irradiation of 7-dehydrocholesterol.[14]
Chemically, the various forms of vitamin D are secosteroids, i.e., steroids in which one of the bonds in the steroid rings is broken.[14] The structural difference between vitamin D2 and vitamin D3 is the side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24.
Deficiency
A diet deficient in vitamin D in conjunction with inadequate sun exposure causes osteomalacia (or rickets when it occurs in children), which is a softening of the bones. In the developed world, this is a rare disease.[15][16] However, vitamin D deficiency has become a worldwide problem in the elderly and remains common in children and adults.[17][18] Low blood calcifediol (25-hydroxy-vitamin D) can result from avoiding the sun.[19] Deficiency results in impaired bone mineralization and bone damage which leads to bone-softening diseases,[20][21] including rickets and osteomalacia.
Rickets
Rickets, a childhood disease, is characterized by impeded growth and soft, weak, deformed long bones that bend and bow under their weight as children start to walk. This condition is characterized by bow legs,[21] which can be caused by calcium or phosphorus deficiency, as well as a lack of vitamin D; today, it is largely found in low-income countries in Africa, Asia, or the Middle East[22] and in those with genetic disorders such as pseudovitamin D deficiency rickets.[23] Maternal vitamin D deficiency may cause overt bone disease from before birth and impairment of bone quality after birth.[24][25] Nutritional rickets exists in countries with intense year-round sunlight such as Nigeria and can occur without vitamin D deficiency.[26][27] Although rickets and osteomalacia are now rare in Britain, outbreaks have happened in some immigrant communities in which osteomalacia sufferers included women with seemingly adequate daylight outdoor exposure wearing Western clothing.[28] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish, and eggs, and low intakes of high-extraction cereals.[29][30][31] The dietary risk factors for rickets include abstaining from animal foods.[28][32] Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate sun exposure. In sunny countries such as Nigeria, South Africa, and Bangladesh, where the disease occurs among older toddlers and children, it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products.[31] Rickets was formerly a major public health problem among the US population; in Denver, where ultraviolet rays are about 20% stronger than at sea level on the same latitude,[33] almost two-thirds of 500 children had mild rickets in the late 1920s.[34] An increase in the proportion of animal protein[32][35] in the 20th century American diet coupled with increased consumption of milk[36][37] fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[38] Also, in the United States and Canada, vitamin D-fortified milk, infant vitamin supplements, and vitamin supplements have helped to eradicate the majority of cases of rickets for children with fat malabsorption conditions.[21]
Osteomalacia
Osteomalacia is a disease in adults that results from vitamin D deficiency. Characteristics of this disease are softening of the bones, leading to bending of the spine, bowing of the legs, proximal muscle weakness, bone fragility, and increased risk for fractures.[39] Osteomalacia reduces calcium absorption and increases calcium loss from bone, which increases the risk for bone fractures. Osteomalacia is usually present when 25-hydroxyvitamin D levels are less than about 10 ng/mL.[1] Although the effects of osteomalacia are thought to contribute to chronic musculoskeletal pain,[40] there is no persuasive evidence of lower vitamin D levels in chronic pain sufferers[41] or that supplementation alleviates chronic nonspecific musculoskeletal pain.[42]
Diabetes
A systematic review of 2014 concluded that the available studies show no evidence of vitamin D3 supplementation having an effect on glucose homeostasis or diabetes prevention.[43] A review article of 2016 reported that while there is increasing evidence that Vitamin D deficiency may be a risk factor for diabetes, over-all evidence regarding vitamin D levels and diabetes mellitus is contradictory, requiring further studies.[44]
Skin pigmentation
Some research shows dark-skinned people living in temperate climates have lower vitamin D levels.[45][46] Dark-skinned people may be less efficient at making vitamin D because melanin in the skin hinders vitamin D synthesis; however, a recent study has found novel evidence that low vitamin D levels among Africans may be due to other reasons.[47] Recent evidence implicates parathyroid hormone in adverse cardiovascular outcomes. Black women have an increase in serum parathyroid hormone at a lower 25(OH)D level than white women.[48] A large-scale association study of the genetic determinants of vitamin D insufficiency in Caucasians found no links to pigmentation.[49][50]
Excess
Vitamin D toxicity is rare.[18] It is caused by supplementing with high doses of vitamin D rather than sunlight. The threshold for vitamin D toxicity has not been established; however, according to some research, the tolerable upper intake level (UL) is 4,000 IU/day for ages 9–71,[51] while other research concludes that, in healthy adults, sustained intake of more than 1250 μg/day (50,000 IU) can produce overt toxicity after several months and can increase serum 25-hydroxyvitamin D levels to 150 ng/ml and greater.[18][52] Those with certain medical conditions, such as primary hyperparathyroidism,[53] are far more sensitive to vitamin D and develop hypercalcemia in response to any increase in vitamin D nutrition, while maternal hypercalcemia during pregnancy may increase fetal sensitivity to effects of vitamin D and lead to a syndrome of mental retardation and facial deformities.[53][54]
A review published in 2015 noted that adverse effects have been reported only at 25(OH)D serum concentrations above 200 nmol/L.[55]
Published cases of toxicity involving hypercalcemia in which the vitamin D dose and the 25-hydroxy-vitamin D levels are known all involve an intake of ≥40,000 IU (1,000 μg) per day.[53]
Research has indicated that Vitamin D toxicity is closely related to a depletion of Vitamin K[56] and that repletion of Vitamin K allows individuals to supplement with higher doses of Vitamin D without the negative calcium-related side effects.
Pregnant or breastfeeding women should consult a doctor before taking a vitamin D supplement. The FDA advised manufacturers of liquid vitamin D supplements that droppers accompanying these products should be clearly and accurately marked for 400 international units (IU). In addition, for products intended for infants, the FDA recommends the dropper hold no more than 400 IU.[57] For infants (birth to 12 months), the tolerable upper limit (maximum amount that can be tolerated without harm) is set at 25 μg/day (1,000 IU). One thousand micrograms per day in infants has produced toxicity within one month.[52] After being commissioned by the Canadian and American governments, the Institute of Medicine (IOM) as of 30 November 2010[update], has increased the tolerable upper limit (UL) to 2,500 IU per day for ages 1–3 years, 3,000 IU per day for ages 4–8 years and 4,000 IU per day for ages 9–71+ years (including pregnant or lactating women).[51]
Effect of excess
Vitamin D overdose causes hypercalcemia, which is a strong indication of vitamin D toxicity – this can be noted with an increase in urination and thirst. If hypercalcemia is not treated, it results in excess deposits of calcium in soft tissues and organs such as the kidneys, liver, and heart, resulting in pain and organ damage.[18][21][39]
The main symptoms of vitamin D overdose which can occur are those of hypercalcemia:
- anorexia
- nausea
- vomiting
This is frequently followed by:
- polyuria
- polydipsia
- weakness
- insomnia
- nervousness
- pruritus
- ultimately renal failure
Furthermore, proteinuria, urinary casts, azotemia, and metastatic calcification (especially in the kidneys) may develop.[52]
Other symptoms of vitamin D toxicity include mental retardation in young children, abnormal bone growth and formation, diarrhea, irritability, weight loss, and severe depression.[18][39]
Vitamin D toxicity is treated by discontinuing vitamin D supplementation and restricting calcium intake. Kidney damage may be irreversible. Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity. The concentrations of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D produced is degraded.[53]
Recommended serum levels
Recommendations on recommended 25(OH)D serum levels vary across authorities, and probably vary based on factors like age.[12]
A 2014 review concluded that the most advantageous serum levels for 25(OH)D appeared to be close to 75 nmol/l.[58] A 2015 review reported that regarding optimal levels, a review of 2004 had recommended that at least 70 nmol/L should be maintained in order to avoid negative health effects, that desirable 25(OH)D levels between 90-120 nmol/l have been reported by another review, but that optimal vitamin D levels are still controversial. The review concluded that ranges from 75 to 100 nmol/L were to be recommended for athletes.[55] Part of the controversy stems from that that numerous studies have found differences in serum levels of 25(OH)D between ethnic groups[59][60] and studies point to genetical as well as environmental to be the reasons behind these variations.[61][62][63][64] Supplementation to achieve these standard levels could cause harmful vascular calcification.[65]
US labs generally report 25(OH)D levels as ng/ml. Other countries often use nmol/l.
In 2011 an IOM committee concluded a serum 25-hydroxyvitamin D level of 20 ng/ml (50 nmol/l) is desirable for bone and overall health. The dietary reference intakes for vitamin D are chosen with a margin of safety and 'overshoot' the targeted serum value to ensure the specified levels of intake achieve the desired serum 25-hydroxyvitamin D levels in almost all persons. No contributions to serum 25-hydroxyvitamin D level are assumed from sun exposure and the recommendations are fully applicable to people with dark skin or negligible exposure to sunlight.[66]
The Institute found serum 25-hydroxyvitamin D concentrations above 30 ng/ml (75 nmol/l) are "not consistently associated with increased benefit". Serum 25-hydroxyvitamin D levels above 50 ng/ml (125 nmol/l) may be cause for concern.[66] However, the desired range of serum 25-hydroxyvitamin D is between 20 and 50 ng/ml.[66]
The risk of cardiovascular disease is lower when vitamin D ranged from 8 to 24 ng/ml (20 to 60 nmol/l). A "threshold effect" appears to occur once a level of 24 ng/ml (60 nmol/l) has been reached i.e., levels of vitamin D over 24 ng/ml (60 nmol/l) did not show added benefit.[67]
Health effects of supplementation
The effects of vitamin D supplementation on health are uncertain.[6][68] A 2013 review did not find any effect from supplementation on the rates of disease, other than a tentative decrease in mortality in the elderly.[69] Low vitamin D levels may result from disease rather than cause disease.[69]
A United States Institute of Medicine report states: "Outcomes related to cancer, cardiovascular disease and hypertension, and diabetes and metabolic syndrome, falls and physical performance, immune functioning and autoimmune disorders, infections, neuropsychological functioning, and preeclampsia could not be linked reliably with calcium or vitamin D intake and were often conflicting."[66]: 5 Some researchers claim the IOM was too definitive in its recommendations and made a mathematical mistake when calculating the blood level of vitamin D associated with bone health.[70] Members of the IOM panel maintain that they used a "standard procedure for dietary recommendations" and that the report is solidly based on the data. Research on vitamin D supplements, including large-scale clinical trials, is continuing.[70]
A 2014 meta-analysis of more than 100 randomized control trials (RCT) led to the conclusion that vitamin D supplements do not alter the outcomes for myocardial infarction, stroke or cerebrovascular disease, cancer or bone fractures.[71] One RCT demonstrated that a daily regimen of 1000 IU of supplemental vitamin D did not affect the risk of colon cancer or recurrent adenomas in people with a background of color adenomas.[citation needed]
Mortality
Vitamin D3 supplementation has been tentatively found to lead to a reduced risk of death in the elderly,[7][69] but the effect has not been deemed pronounced or certain enough to make taking supplements recommendable.[8]
Other forms (Vitamin D2, alfacalcidol, and calcitriol) do not appear to have any beneficial effects with regard to the risk of death.[7] High blood levels appear to be associated with a lower risk of death, but it is unclear if supplementation can result in this benefit.[72] Both an excess and a deficiency in vitamin D appear to cause abnormal functioning and premature aging.[73][74][75] The relationship between serum calcifediol level and all-cause mortality is parabolic.[66] Harm from vitamin D appears to occur at a lower vitamin D level in the black population than in the white population.[66]: 435
Bone health
In general, no good evidence supports the commonly held belief that vitamin D supplements can help prevent osteoporosis.[8] Its general use for prevention of this disease in those without vitamin D deficiency is thus likely not needed.[76]
For older people with osteoporosis, taking vitamin D with calcium may help prevent hip fractures, but it also slightly increases the risk of stomach and kidney problems.[77] Supplementation with higher doses of vitamin D, in those older than 65 years, may decrease fracture risk.[78] This appears to apply more to people in institutions than those living independently.[79]
Vitamin D deficiency causes osteomalacia (called rickets when it occurs in children). Use of vitamin D in children with normal vitamin D levels does not appear to improve bone density.[80] Beyond that, low serum vitamin D levels have been associated with falls, and low bone mineral density.[81] Taking extra vitamin D, however, does not appear to change the risk.[82]
Because it found mounting evidence for a benefit to bone health, though it had not found good evidence of other benefits, the Food and Drug Administration of the United States has proposed requiring manufacturers to declare the amount of vitamin D on nutrition facts labels, as "nutrients of public health significance". As of August 2015, this is currently still open for public comment.[83]
Athletes who are vitamin D deficient are at an increased risk of stress fractures and/or major breaks, particularly those engaging in contact sports. The greatest benefit with supplementation is seen in athletes who are deficient (25(OH)D serum levels <30 ng/ml), or severely deficient (25(OH)D serum levels <25 ng/ml). Incremental decreases in risks are observed with rising serum 25(OH)D concentrations plateauing at 50 ng/ml with no additional benefits seen in levels beyond this point.[84]
Cancer
Vitamin D supplements have been widely marketed for their claimed anticancer properties.[85] Associations have been shown in observational studies between low vitamin D levels and the risk of development of certain cancers including colon cancer.[86][87]
It is unclear, however, if taking additional vitamin D in the diet or as supplements affects the risk of cancer. Reviews have described the evidence as being "inconsistent, inconclusive as to causality, and insufficient to inform nutritional requirements"[66] and "not sufficiently robust to draw conclusions".[88]
A 2014 review found that supplements had no significant effect on cancer risk.[8] Another review suggested that vitamin D3 may slightly decrease the risk of death from cancer (one fewer death in 150 people over 5 years), but concerns with the quality of the data were noted.[89]
Insufficient evidence exists to recommend vitamin D supplements for people with cancer, although some evidence suggests hypovitaminosis D may be associated with a worse outcome for some cancers,[90] and that higher 25-hydroxy vitamin D levels at the time of diagnosis are associated with better outcomes.[91]
Cardiovascular disease
Taking vitamin D supplements does not meaningfully reduce the risk of stroke, cerebrovascular disease, cardial infarction, or ischaemic heart disease.[8] Supplementation has no effect on blood pressure.[92]
Depression
Clinical trials of vitamin D supplementation for depressive symptoms have generally been of low quality and show no overall effect, although subgroup analysis showed supplementation for participants with clinically significant depressive symptoms or depressive disorder had a moderate effect.[93]
Cognition and dementia
A systematic review of clinical studies shows an association between low vitamin D levels, cognitive impairment, and a higher risk of developing Alzheimer's disease. However, lower vitamin D concentrations is also associated with poor nutrition and spending less time outdoors. Therefore, alternative explanations for the increase in cognitive impairment exist and hence a direct causal relationship between vitamin D levels and cognition could not be established.[94]
Immune system
Infectious disease
In general, vitamin D functions to activate the innate and dampen the adaptive immune systems.[95] Deficiency has been linked to increased risk of viral infections, including HIV and influenza.[96][97][98] Low levels of vitamin D appear to be a risk factor for tuberculosis,[99] and historically it was used as a treatment.[100] Evidence is lacking on whether vitamin D reduces risk of respiratory infections in children under five years of age.[101] No clinical trial has been done to assess its effect on preventing other infections such as TB and malaria.
Autoimmune disease
Although tentative data link low levels of vitamin D to asthma, evidence to support a beneficial effect from supplementation is inconclusive.[102] Accordingly, supplementation is not currently recommended for treatment or prevention of asthma.[103]
Vitamin D hypovitaminosis may be a risk factor for multiple sclerosis,[104] but no evidence indicates vitamin D has any clinically significant benefit as a treatment.[105] Further research is needed to determine if the association represents a cause and effect relationship.[106]
Low levels of vitamin D are associated with Crohn's disease and ulcerative colitis.[107] Further studies are required to determine its significance.[107]
Pregnancy
Low levels of vitamin D in pregnancy are associated with gestational diabetes, pre-eclampsia, and small infants.[108] The benefit of supplements, however, is unclear.[108] Pregnant women who take an adequate amount of vitamin D during gestation may experience positive immune effects.[109] Pregnant women often do not take the recommended amount of vitamin D.[109]
Weight loss
Though hypothesized that vitamin D supplementation may be an effective treatment for obesity apart from calorie restriction, one systematic review found no association of supplementation with body weight or fat mass.[110] A 2016 meta-analysis found that circulating vitamin D status was improved by weight loss, indicating that fat mass may be inversely associated with blood levels of vitamin D.[111]
Mechanism of action
Metabolic activation
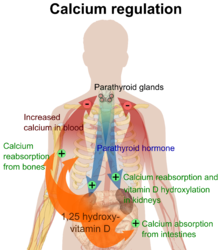


Vitamin D is carried in the bloodstream to the liver, where it is converted into the prohormone calcifediol. Circulating calcifediol may then be converted into calcitriol, the biologically active form of vitamin D, in the kidneys. Following the final converting step in the kidney, calcitriol is released into the circulation. By binding to vitamin D-binding protein, a carrier protein in the plasma, calcitriol is transported to various target organs.[14] In addition to the kidneys, calcitriol is also synthesized by monocyte-macrophages in the immune system. When synthesized by monocyte-macrophages, calcitriol acts locally as a cytokine, defending the body against microbial invaders by stimulating the innate immune system.[113]
Whether it is made in the skin or ingested, cholecalciferol is hydroxylated in the liver at position 25 (upper right of the molecule) to form 25-hydroxycholecalciferol (calcifediol or 25(OH)D). This reaction is catalyzed by the microsomal enzyme vitamin D 25-hydroxylase,[114] which is produced by hepatocytes. Once made, the product is released into the plasma, where it is bound to an α-globulin, vitamin D-binding protein.[115]
Calcifediol is transported to the proximal tubules of the kidneys, where it is hydroxylated at the 1-α position (lower right of the molecule) to form calcitriol (1,25-dihydroxycholecalciferol and abbreviated to 1,25(OH)2D). This product is a potent ligand of the vitamin D receptor, which mediates most of the physiological actions of the vitamin. The conversion of calcifediol to calcitriol is catalyzed by the enzyme 25-hydroxyvitamin D3 1-alpha-hydroxylase, the levels of which are increased by parathyroid hormone (and additionally by low calcium or phosphate).
Biosynthesis
This section needs expansion. You can help by adding to it. (February 2015) |
In the presence of UV radiation, many animals synthesize vitamin D3 from 7-dehydrocholesterol, and many fungi synthesize vitamin D2 from ergosterol.
Photochemistry


The transformation that converts 7-dehydrocholesterol to vitamin D3 occurs in two steps.[116][117] First, 7-dehydrocholesterol is photolyzed by ultraviolet light in a 6-electron conrotatory ring-opening electrocyclic reaction; the product is previtamin D3. Second, previtamin D3 spontaneously isomerizes to vitamin D3 (cholecalciferol) in an antarafacial sigmatropic [1,7] hydride shift. At room temperature, the transformation of previtamin D3 to vitamin D3 in an organic solvent takes about 12 days to complete. The conversion of previtamin D3 to vitamin D3 in the skin is about 10 times faster than in an organic solvent [118]
Evolution
Photosynthesis of vitamin D in the ocean by phytoplankton (such as coccolithophore and Emiliania huxleyi) has existed for more than 500 million years and continues to the present. Although primitive vertebrates in the ocean could absorb calcium from the ocean into their skeletons and eat plankton rich in vitamin D, land animals required another way to satisfy their vitamin D requirement for a calcified skeleton without relying on plants. Land vertebrates have been making their own vitamin D for more than 350 million years.[119]
Vitamin D can be synthesized only by a photochemical process, so land vertebrates had to ingest foods that contained vitamin D or had to be exposed to sunlight to photosynthesize vitamin D in their skin to satisfy their vitamin D requirements.[118]
Synthesis in the skin

Vitamin D3 is produced photochemically from 7-dehydrocholesterol in the skin of most vertebrate animals, including humans.[120] The precursor of vitamin D3, 7-dehydrocholesterol is produced in relatively large quantities. 7-Dehydrocholesterol reacts with UVB light at wavelengths between 270 and 300 nm, with peak synthesis occurring between 295 and 297 nm.[121] These wavelengths are present in sunlight, as well as in the light emitted by the UV lamps in tanning beds (which produce ultraviolet primarily in the UVA spectrum, but typically produce 4% to 10% of the total UV emissions as UVB). Exposure to light through windows is insufficient because glass almost completely blocks UVB light.[122][123]
Adequate amounts of vitamin D can be produced with moderate sun exposure to the face, arms and legs, averaging 5–30 minutes twice per week, or approximately 25% of the time for minimal sunburn. The darker the skin, and the weaker the sunlight, the more minutes of exposure are needed. Vitamin D overdose is impossible from UV exposure; the skin reaches an equilibrium where the vitamin degrades as fast as it is created.[18][124][125]
Sunscreen absorbs or reflects ultraviolet light and prevents much of it from reaching the skin. Sunscreen with a sun protection factor (SPF) of 8 based on the UVB spectrum decreases vitamin D synthetic capacity by 95%, and SPF 15 decreases it by 98%.[126]
The skin consists of two primary layers: the inner layer called the dermis, composed largely of connective tissue, and the outer, thinner epidermis. Thick epidermis in the soles and palms consists of five strata; from outer to inner, they are: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale. Vitamin D is produced in the two innermost strata, the stratum basale and stratum spinosum.
The naked mole-rat appears to be naturally cholecalciferol-deficient, as serum 25-OH vitamin D levels are undetectable.[127] In some animals, the presence of fur or feathers blocks the UV rays from reaching the skin. In birds and fur-bearing mammals, vitamin D is generated from the oily secretions of the skin deposited onto the feathers or fur and is obtained orally during grooming.[128]
Biological activity
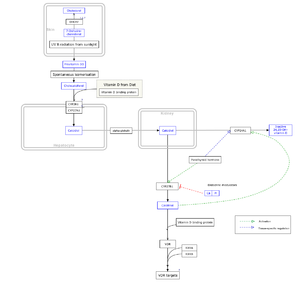
The active vitamin D metabolite calcitriol mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.[14] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.[129] The vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDRs are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.[38]
One of the most important roles of vitamin D is to maintain skeletal calcium balance by promoting calcium absorption in the intestines, promoting bone resorption by increasing osteoclast number, maintaining calcium and phosphate levels for bone formation, and allowing proper functioning of parathyroid hormone to maintain serum calcium levels. Vitamin D deficiency can result in lower bone mineral density and an increased risk of reduced bone density (osteoporosis) or bone fracture because a lack of vitamin D alters mineral metabolism in the body.[130] Thus, although this may initially appear paradoxical, vitamin D is also critical for bone remodeling through its role as a potent stimulator of bone resorption.[130]
The VDR may be involved in cell proliferation and differentiation. Vitamin D also affects the immune system, and VDRs are expressed in several white blood cells, including monocytes and activated T and B cells.[131] In vitro, vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells, and affects the synthesis of neurotrophic factors, nitric oxide synthase, and glutathione.[132]
Apart from VDR activation, various alternative mechanisms of action are under study, such as inhibition of signal transduction by hedgehog, a hormone involved in morphogenesis.[133]
History
American researchers Elmer McCollum and Marguerite Davis in 1914[4] discovered a substance in cod liver oil which later was called "vitamin A". British doctor Edward Mellanby noticed dogs that were fed cod liver oil did not develop rickets and concluded vitamin A, or a closely associated factor, could prevent the disease. In 1922, Elmer McCollum tested modified cod liver oil in which the vitamin A had been destroyed.[4] The modified oil cured the sick dogs, so McCollum concluded the factor in cod liver oil which cured rickets was distinct from vitamin A. He called it vitamin D because it was the fourth vitamin to be named.[134][135][136] It was not initially realized that, unlike other vitamins, vitamin D can be synthesised by humans through exposure to UV light.
In 1925,[4] it was established that when 7-dehydrocholesterol is irradiated with light, a form of a fat-soluble vitamin is produced (now known as D3). Alfred Fabian Hess stated: "Light equals vitamin D."[137] Adolf Windaus, at the University of Göttingen in Germany, received the Nobel Prize in Chemistry in 1928 for his work on the constitution of sterols and their connection with vitamins.[138] In 1929, a group at NIMR in Hampstead, London, were working on the structure of vitamin D, which was still unknown, as well as the structure of steroids. A meeting took place with J.B.S. Haldane, J.D. Bernal, and Dorothy Crowfoot to discuss possible structures, which contributed to bringing a team together. X-ray crystallography demonstrated the sterol molecules were flat, not as proposed by the German team led by Windaus. In 1932, Otto Rosenheim and Harold King published a paper putting forward structures for sterols and bile acids which found immediate acceptance.[139] The informal academic collaboration between the team members Robert Benedict Bourdillon, Otto Rosenheim, Harold King, and Kenneth Callow was very productive and led to the isolation and characterization of vitamin D.[140] At this time, the policy of the Medical Research Council was not to patent discoveries, believing the results of medical research should be open to everybody. In the 1930s, Windaus clarified further the chemical structure of vitamin D.[141]
In 1923, American biochemist Harry Steenbock at the University of Wisconsin demonstrated that irradiation by ultraviolet light increased the vitamin D content of foods and other organic materials.[142] After irradiating rodent food, Steenbock discovered the rodents were cured of rickets. A vitamin D deficiency is a known cause of rickets. Using $300 of his own money, Steenbock patented his invention. His irradiation technique was used for foodstuffs, most memorably for milk. By the expiration of his patent in 1945, rickets had been all but eliminated in the US.[143]
In 1971–72, the further metabolism of vitamin D to active forms was discovered. In the liver, vitamin D was found to be converted to calcifediol. Calcifediol is then converted by the kidneys to calcitriol, the biologically active form of vitamin D.[11] Calcitriol circulates as a hormone in the blood, regulating the concentration of calcium and phosphate in the bloodstream and promoting the healthy growth and remodeling of bone. The vitamin D metabolites, calcifediol and calcitriol, were identified by competing teams led by Michael F. Holick in the laboratory of Hector DeLuca and by Tony Norman and colleagues.[144][145][146]
Guidelines
Dietary reference intakes
Different institutions propose different recommendations concerning daily amounts of the vitamin.The recommended daily intake of vitamin D may not be sufficient if sunlight exposure is limited.[147]
(Conversion : 1 µg = 40 IU and 0.025 µg = 1 IU)[148]
Australia and New Zealand
About a third of Australians have vitamin D deficiency.[149] Australia and New Zealand have established guidelines for dietary vitamin D intake as follows:[150]
| Age group | Adequate Intake (μg) | Upper Level of Intake (μg) |
|---|---|---|
| Infants 0–12 months | 5.0 | 25.0 |
| Children 1–18 years | 5.0 | 80.0 |
| Adults 19–50 years | 5.0 | 80.0 |
| Adults 51–70 years | 10.0 | 80.0 |
| Adults > 70 years | 15.0 | 80.0 |
Canada
According to Health Canada[151] the recommended dietary allowances (RDA) for vitamin D are:
| Age group | RDA (IU) | Tolerable upper intake (IU) |
|---|---|---|
| Infants 0–6 months | 400* | 1,000 |
| Infants 7–12 months | 400* | 1,500 |
| Children 1–3 years | 600 | 2,500 |
| Children 4–8 years | 600 | 3,000 |
| Children and Adults 9–70 years | 600 | 4,000 |
| Adults > 70 years | 800 | 4,000 |
| Pregnancy & Lactation | 600 | 4,000 |
Note*: Adequate intake rather than recommended dietary allowance
European Union
The recommended daily amount for vitamin D in the European Union is 5 µg.[152] In 2012, the German Society for Nutrition, a private organisation, increased the recommended daily amount to 20 µg.[153]
The European Menopause and Andropause Society recommended 15 µg (600 IU) until age 70, and 20 µg (800 IU) in older than 71 years, in postmenopausal women. This dose should be increased to 4,000 IU/day in some patients with very low vitamin D status or in case of comorbid conditions.[154]
The UK National Health Service recommends babies and young children aged six months to five years, pregnant or breastfeeding women, and sun-deprived elderly people should take daily vitamin supplements to ensure sufficient vitamin D intake.[155] In July 2016, Public Health England recommended that everyone consider taking a daily supplement containing 10 µg of vitamin D during autumn and winter because of inadequate sunlight for vitamin D synthesis.[156]
United States
According to the United States Institute of Medicine,[66] the recommended dietary allowances (RDA) of vitamin D are:
| Age group | RDA (IU/day) |
|---|---|
| Infants 0–6 months | 400* |
| Infants 6–12 months | 400* |
| 1–70 years | 600 (15 μg/day) |
| 71+ years | 800 (20 μg/day) |
| Pregnant/Lactating | 600 (15 μg/day) |
- Asterisk for infants indicates adequate intake (AI) for infants, as an RDA has yet to be established for infants.[66]
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For vitamin D labeling purposes 100% of the Daily Value was 400 IU (10 μg), but as of May 2016 it has been revised to 800 IU (20 μg). A table of the pre-change adult Daily Values is provided at Reference Daily Intake. Food and supplement companies have until July 28, 2018 to comply with the change.
Upper intake levels
The tolerable upper intake level is defined as "the highest average daily intake of a nutrient that is likely to pose no risk of adverse health effects for nearly all persons in the general population.[66]: 403 " Although tolerable upper intake levels are believed to be safe, information on the long-term effects is incomplete and these levels of intake are not recommended:[66]: 403 : 433
| Age group | Tolerable upper intake level |
|---|---|
| Infants 0–6 months | 1,000 IU/day (25 µg/day) |
| Infants 6–12 months | 1,500 IU/day (37.5 µg/day) |
| 1–3 years | 2,500 IU/day (62.5 µg/day) |
| 4–8 years | 3,000 IU/day (75 µg/day) |
| 9+ years | 4,000 IU/day (100 µg/day) |
| Pregnant/lactating | 4,000 IU/day[66]: 5 (100 µg/day) |
The dietary reference intake for vitamin D issued by the Institute of Medicine (IOM) in 2010 superseded a previous recommendation which had adequate intake status. The recommendations were formed assuming the individual has no skin synthesis of vitamin D because of inadequate sun exposure. The reference intake for vitamin D refers to total intake from food, beverages and supplements, is intended for the North American population, and assumes that calcium requirements are being met.[66]: 5
One school of thought contends the human physiology is fine-tuned to an intake of 4,000–12,000 IU/day from sun exposure with concomitant serum 25-hydroxyvitamin D levels of 40 to 80 ng/ml[157] and this is required for optimal health. Proponents of this view, who include some members of the panel that drafted a now-superseded 1997 report on vitamin D from the IOM, contend the IOM's warning about serum concentrations above 50 ng/ml lacks biological plausibility. They suggest, for some people, reducing the risk of preventable disease requires a higher level of vitamin D than that recommended by the IOM.[157][158]
According to the European Food Safety Authority, the tolerable upper intake levels[159] are:
- 0–12 months: 25 µg/day (1,000 IU)
- 1–10 years: 50 µg/day (2,000 IU)
- 11–17 years: 100 µg/day (4,000 IU)
- 17+: 100 µg/day (4,000 IU)
- Pregnant/lactating women: 100 µg/day (4,000 IU)
Allowable health claims
Apart from the above discussion on health effects or scientific evidence for lowering disease risk, governmental regulatory agencies stipulate for the food industry health claims allowable as statements on packaging.
European Food Safety Authority (EFSA)[160]
- normal function of the immune system
- normal inflammatory response
- normal muscle function
- reduced risk of falling in people over age 60[161]
US Food and Drug Administration (FDA)
- may reduce the risk of osteoporosis[162]
- adequate calcium and regular exercise may help to achieve strong bones in children and adolescents and may reduce the risk of osteoporosis in older adults. An adequate intake of vitamin D is also necessary[163]
Other possible agencies with claim guidance: Japan FOSHU[164] and Australia-New Zealand.[165]
Dietary sources
Vitamin D is found in few dietary sources.[1][3][18][21] Sunlight exposure is the primary source of vitamin D for the majority of people, other than supplements.[2]
While some studies have found that vitamin D3 raises 25(OH)D blood levels faster and remains active in the body longer,[166][167] others contend that vitamin D2 sources are equally bioavailable and effective as D3 for raising and sustaining 25(OH)D.[168][169][170]
Vitamin D2
Mushrooms
Mushrooms are a good dietary source of vitamin D2. They contain high concentrations of ergosterol (provitamin D2), and sunlight or ultraviolet radiation triggers its conversion to viosterol (previtamin D2), which then turns into vitamin D2. Low values in mushrooms for vitamin D2 below indicate no or only incidental exposure to sunlight. When fresh mushrooms or dried powders are purposely exposed to artificial sunlight by use of an industrial ultraviolet lamp, vitamin D2 levels can be concentrated to much higher levels.[168][171][172]
Content of vitamin D2 per 100g:[173]
- Mushrooms, portobello, exposed to ultraviolet light, raw: Vitamin D2: 11.2 μg (446 IU)
- Mushrooms, portobello, exposed to ultraviolet light, grilled: Vitamin D2: 13.1 μg (524 IU)
- Mushrooms, shiitake, dried: Vitamin D2: 3.9 μg (154 IU)
- Mushrooms, shiitake, raw: Vitamin D2: 0.4 μg (18 IU)
- Mushrooms, portobello, raw: Vitamin D2: 0.3 μg (10 IU)
Human bioavailability of vitamin D2 from vitamin D2-enhanced button mushrooms via UV-B irradiation is effective in improving vitamin D status and not different from a vitamin D2 supplement.[168][174] Vitamin D2 from UV-irradiated yeast baked into bread or mushrooms is bioavailable and increases blood levels of 25(OH)D.[168]
By visual assessment or using a chromometer, no significant discoloration of irradiated mushrooms, as measured by the degree of "whiteness", was observed.[175] Claims have been made that a normal serving (approx. 3 oz or 1/2 cup, or 60 grams) of fresh mushrooms treated with ultraviolet light have increased vitamin D content to levels up to 80 micrograms or 2700 IU if exposed to just 5 minutes of UV light after being harvested.[171]
Plants
- Alfalfa (Medicago sativa subsp. sativa), shoot: 4.8 μg (192 IU) vitamin D2, 0.1 μg (4 IU) vitamin D3 (per 100 g).[176]
Vitamin D3
In some countries, staple foods are artificially fortified with vitamin D.[177]
- Vegan sources
- Lichen
- Cladina arbuscula specimens grown under different natural conditions: The contents of vitamin D3 range from 0.67 to 2.04 μg g⁻¹ dry matter in the thalli of C. arbuscula specimens grown under different natural conditions.[178]
- Lichen
- Animal sources[173]
- Fish liver oils, such as cod liver oil, 4.5 g (1 teaspoon) provides 450 IU (100 IU/g)
- Fatty fish species, such as:
- Salmon, pink, cooked, dry heat, 100 grams (3.5 oz): 522 IU (5.2 IU/g)
- Mackerel, Pacific and jack, mixed species, cooked, dry heat, 100 grams (3.5 oz): 457 IU (4.6 IU/g)
- Tuna, canned in oil, 100 grams (3.5 oz): 269 IU (2.7 IU/g)
- Sardines, canned in oil, drained, 100 grams (3.5 oz): 193 IU (1.9 IU/g)
- Cooked egg yolk: 44 IU for a 61 g egg (0.7 IU/g)
- Beef liver, cooked, braised, 100 grams (3.5 oz): 49 IU (0.5 IU/g)
Industrial production
Vitamin D3 (cholecalciferol) is produced industrially by exposing 7-dehydrocholesterol to UVB light, followed by purification.[179] The 7-dehydrocholesterol is a natural substance in fish organs, especially the liver,[180] or in wool grease (lanolin) from sheep. Vitamin D2 (ergocalciferol) is produced in a similar way using ergosterol from yeast or mushrooms as a starting material.[168][179]
Effects of cooking
Vitamin D content in typical foods is reduced variably by cooking, such as by boiling, frying or baking.[181] Boiled, fried and baked foods retained 69–89% of original vitamin D.[181]
Research and education
A 2016 review stated that reports of favorable health outcomes deriving from moderate sun exposure and adequate vitamin D concentration or vitamin D supplementation were not adequately described in the medical literature.[182] While medical authorities in the United States have recommended that men, women and children reduce their sun exposure based on concerns about skin cancer, the majority of Americans have vitamin D deficiency that may lead to increased risk of diseases possibly preventable by adequate sun exposure and the resulting higher vitamin D levels.[182] The review proposed various potential health benefits from sun exposure and improved vitamin D status, including the potential for reduced risk of different types of cancer, cardiovascular diseases, cognitive disorders, myopia and macular degeneration, diabetes and multiple sclerosis, all of which are being evaluated in clinical trials.[182][183][184] The ongoing United States National Health and Nutrition Examination Survey (NHANES)[185] is assessing the prevalence of numerous diseases possibly associated with vitamin D deficiency.[186] Starting in 2014, the United States National Institutes of Health and Office of Dietary Supplements established a Vitamin D Initiative to track current research and provide education to consumers.[187]
European research is assessing vitamin D intake levels in association with disease incidence and policies for judicious sun exposure, dietary recommendations, food fortification, and vitamin D supplementation.[188]
Preliminary studies in overweight or obese soldiers[189] and children[190][191][192] established an association that blood levels of vitamin D are proportionately suppressed by fat mass, indicating a need to develop specific clinical screening and treatment guidance for these populations.
References
- ^ a b c d Holick MF (March 2006). "High prevalence of vitamin D inadequacy and implications for health". Mayo Clin. Proc. 81 (3): 353–73. doi:10.4065/81.3.353. PMID 16529140.
- ^ a b Calvo MS, Whiting SJ, Barton CN; Whiting; Barton (February 2005). "Vitamin D intake: a global perspective of current status". J. Nutr. 135 (2): 310–6. PMID 15671233.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Norman AW (August 2008). "From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health". Am. J. Clin. Nutr. 88 (2): 491S–499S. PMID 18689389.
- ^ a b c d Wolf G (June 2004). "The discovery of vitamin D: the contribution of Adolf Windaus". J Nutr. 134 (6): 1299–302. PMID 15173387.
- ^ Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM; Chung; Trikalinos; Mitri; Brendel; Patel; Lichtenstein; Lau; Balk (March 2010). "Vitamin D and Cardiometabolic Outcomes: A Systematic Review". Annals of Internal Medicine. 152 (5): 307–14. doi:10.7326/0003-4819-152-5-201003020-00009. PMC 3211092. PMID 20194237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, Lichtenstein A, Patel K, Raman G, Tatsioni A, Terasawa T, Trikalinos TA; Balk; Brendel; Ip; Lau; Lee; Lichtenstein; Patel; Raman; Tatsioni; Terasawa; Trikalinos (August 2009). "Vitamin D and calcium: a systematic review of health outcomes". Evidence report/technology assessment (183): 1–420. PMC 4781105. PMID 20629479.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C; Gluud; Nikolova; Whitfield; Wetterslev; Simonetti; Bjelakovic; Gluud (2014). "Vitamin D supplementation for prevention of mortality in adults". Cochrane Database Syst Rev (Systematic review). 1 (1): CD007470. doi:10.1002/14651858.CD007470.pub3. PMID 24414552.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Bolland MJ, Grey A, Gamble GD, Reid IR (January 2014). "The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis". Lancet Diabetes Endocrinol (Meta-analysis). 2 (4): 307–20. doi:10.1016/S2213-8587(13)70212-2. PMID 24703049.
- ^ "Vitamin D Tests". Lab Tests Online (USA). American Association for Clinical Chemistry. Retrieved June 23, 2013.
- ^ Hollis BW (January 1996). "Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it". Calcif. Tissue Int. 58 (1): 4–5. doi:10.1007/BF02509538. PMID 8825231.
- ^ a b Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ (1971). "Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine". Biochemistry. 10 (14): 2799–804. doi:10.1021/bi00790a023. PMID 4326883.
- ^ a b "Vitamin D". NIH Office of Dietary Supplements. February 11, 2016. Retrieved December 6, 2016.
- ^ Dorland's Illustrated Medical Dictionary, under Vitamin (Table of Vitamins)
- ^ a b c d "About Vitamin D". University of California, Riverside. November 2011. Retrieved January 24, 2015.
- ^ "Rickets". National Health Service. March 8, 2012. Retrieved July 9, 2012.
- ^ MedlinePlus Encyclopedia: Rickets
- ^ Eriksen EF, Glerup H (2002). "Vitamin D deficiency and aging: implications for general health and osteoporosis". Biogerontology. 3 (1–2): 73–7. doi:10.1023/A:1015263514765. PMID 12014847.
- ^ a b c d e f g Holick MF (July 2007). "Vitamin D deficiency". N. Engl. J. Med. 357 (3): 266–81. doi:10.1056/NEJMra070553. PMID 17634462.
- ^ Schoenmakers I, Goldberg GR, Prentice A (2008). "Abundant sunshine and vitamin D deficiency". British Journal of Nutrition. 99 (6): 1171–3. doi:10.1017/S0007114508898662. PMC 2758994. PMID 18234141.
- ^ Grant WB, Holick MF (2005). "Benefits and requirements of vitamin D for optimal health: a review". Alternative medicine review. 10 (2): 94–111. PMID 15989379.
- ^ a b c d e Brown JE (2008). Nutrition through the life cycle. Belmont, CA: Thomson/Wadsworth. ISBN 0-495-11637-8.
- ^ Lerch C, Meissner T (2007). Lerch, Christian (ed.). "Interventions for the prevention of nutritional rickets in term born children". Cochrane database of systematic reviews (Online) (4): CD006164. doi:10.1002/14651858.CD006164.pub2. PMID 17943890.
- ^ Zargar AH, Mithal A, Wani AI, Laway BA, Masoodi SR, Bashir MI, Ganie MA (June 2000). "Pseudovitamin D deficiency rickets—a report from the Indian subcontinent". Postgraduate Medical Journal. 76 (896): 369–72. doi:10.1136/pmj.76.896.369. PMC 1741602. PMID 10824056.
- ^ Elidrissy AT (2016). "The Return of Congenital Rickets, Are We Missing Occult Cases?". Calcif Tissue Int (Review). 99 (3): 227–36. doi:10.1007/s00223-016-0146-2. PMID 27245342.
- ^ Paterson CR, Ayoub D (2015). "Congenital rickets due to vitamin D deficiency in the mothers". Clin Nutr (Review). 34 (5): 793–8. doi:10.1016/j.clnu.2014.12.006. PMID 25552383.
- ^ Oramasionwu GE, Thacher TD, Pam SD, Pettifor JM, Abrams SA (2008). "Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children". The British journal of nutrition. 100 (2): 387–92. doi:10.1017/S0007114507901233. PMID 18197991.
- ^ Fischer PR, Rahman A, Cimma JP, Kyaw-Myint TO, Kabir AR, Talukder K, Hassan N, Manaster BJ, Staab DB, Duxbury JM, Welch RM, Meisner CA, Haque S, Combs GF (1999). "Nutritional rickets without vitamin D deficiency in Bangladesh". Journal of tropical pediatrics. 45 (5): 291–3. doi:10.1093/tropej/45.5.291. PMID 10584471.
- ^ a b Dunnigan MG, Henderson JB (1997). "An epidemiological model of privational rickets and osteomalacia". The Proceedings of the Nutrition Society. 56 (3): 939–56. doi:10.1079/PNS19970100. PMID 9483661.
- ^ Robertson I, Ford JA, McIntosh WB, Dunnigan MG (1981). "The role of cereals in the aetiology of nutritional rickets: the lesson of the Irish National Nutrition Survey 1943–8". The British journal of nutrition. 45 (1): 17–22. doi:10.1079/BJN19810073. PMID 6970590.
- ^ Clements MR (1989). "The problem of rickets in UK Asians". Journal of Human Nutrition and Dietetics. 2 (2): 105–116. doi:10.1111/j.1365-277X.1989.tb00015.x.
- ^ a b Pettifor JM (2004). "Nutritional rickets: deficiency of vitamin D, calcium, or both?". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1725S–9S. PMID 15585795.
- ^ a b Dunnigan MG, Henderson JB, Hole DJ, Barbara Mawer E, Berry JL (2007). "Meat consumption reduces the risk of nutritional rickets and osteomalacia". British Journal of Nutrition. 94 (6): 983–91. doi:10.1079/BJN20051558. PMID 16351777.
- ^ "US National Institutes Of Health, National Cancer Institute". Science.education.nih.gov. Retrieved August 24, 2010.
- ^ Weick MT (1967). "A history of rickets in the United States". The American Journal of Clinical Nutrition. 20 (11): 1234–41. PMID 4862158.
- ^ Garrison RH, Somer E (1997). The Nutrition Desk Reference. McGraw-Hill. ISBN 978-0-87983-826-3.
- ^ DuPuis EM (2002). Nature's Perfect Food: How Milk Became America's Drink. ISBN 978-0-8147-1938-1.
- ^ Teegarden D, Lyle RM, Proulx WR, Johnston CC, Weaver CM (1999). "Previous milk consumption is associated with greater bone density in young women". The American Journal of Clinical Nutrition. 69 (5): 1014–7. PMID 10232644.
- ^ a b Holick MF (2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1678S–88S. PMID 15585788.
- ^ a b c Insel PM, Turner ER, Ross D (2006). Discovering nutrition (2nd ed.). Boston: Jones and Bartlett Publishers. ISBN 0-7637-3555-8.
- ^ Holick MF (2003). "Vitamin D: A millenium perspective". Journal of Cellular Biochemistry. 88 (2): 296–307. doi:10.1002/jcb.10338. PMID 12520530.
- ^ Straube S, Andrew Moore R, Derry S, McQuay HJ (2009). "Vitamin D and chronic pain". Pain. 141 (1–2): 10–3. doi:10.1016/j.pain.2008.11.010. PMID 19084336.
- ^ Gaikwad M, Vanlint S, Mittinity M, Moseley GL, Stocks N (2016). "Does vitamin D supplementation alleviate chronic nonspecific musculoskeletal pain? A systematic review and meta-analysis". Clin Rheumatol Online. doi:10.1007/s10067-016-3205-1. PMID 26861032.
- ^ Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA (2014). "Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis". The Journal of Clinical Endocrinology and Metabolism (Review). 99 (10): 3551–60. doi:10.1210/jc.2014-2136. PMC 4483466. PMID 25062463.
- ^ Nakashima A, Yokoyama K, Yokoo T, Urashima M (2016). "Role of vitamin D in diabetes mellitus and chronic kidney disease". World Journal of Diabetes (Review). 7 (5): 89–100. doi:10.4239/wjd.v7.i5.89. PMC 4781904. PMID 26981182.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Azmina Govindji RD (July 1, 2010). "When it's sunny, top up your vitamin D". TheIsmaili.org. Retrieved July 1, 2010.
- ^ Ford L, Graham V, Wall A, Berg J (November 2006). "Vitamin D concentrations in an UK inner-city multicultural outpatient population". Annals of Clinical Biochemistry. 43 (6): 468–73. doi:10.1258/000456306778904614. PMID 17132277.
- ^ Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ (2010). "Blood vitamin D levels in relation to genetic estimation of African ancestry". Cancer Epidemiology, Biomarkers & Prevention. 19 (9): 2325–31. doi:10.1158/1055-9965.EPI-10-0482. PMC 2938736. PMID 20647395.
- ^ Aloia JF, Chen DG, Chen H (2010). "The 25(OH)D/PTH Threshold in Black Women". The Journal of Clinical Endocrinology and Metabolism. 95 (11): 5069–73. doi:10.1210/jc.2010-0610. PMC 2968726. PMID 20685862.
- ^ Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD (2010). "Common genetic determinants of vitamin D insufficiency: a genome-wide association study". Lancet. 376 (9736): 180–8. doi:10.1016/S0140-6736(10)60588-0. PMC 3086761. PMID 20541252.
- ^ Bouillon R (2010). "Genetic and environmental determinants of vitamin D status". Lancet. 376 (9736): 148–9. doi:10.1016/S0140-6736(10)60635-6. PMID 20541253.
- ^ a b Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (January 2011). "The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know". J. Clin. Endocrinol. Metab. 96 (1): 53–8. doi:10.1210/jc.2010-2704. PMC 3046611. PMID 21118827.
- ^ a b c Vitamin D at Merck Manual of Diagnosis and Therapy Professional Edition
- ^ a b c d Vieth, R. (1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety" (PDF). The American Journal of Clinical Nutrition. 69 (5): 842–856. PMID 10232622.
- ^ Tolerable Upper Intake Limits for Vitamins And Minerals (PDF). European Food Safety Authority. December 2006. ISBN 92-9199-014-0.
- ^ a b Dahlquist DT, Dieter BP, Koehle MS (2015). "Plausible ergogenic effects of vitamin D on athletic performance and recovery". Journal of the International Society of Sports Nutrition (Review). 12: 33. doi:10.1186/s12970-015-0093-8. PMC 4539891. PMID 26288575.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Masterjohn, C (2007). "Vitamin D toxicity redefined: vitamin K and the molecular mechanism". Med Hypotheses. 68 (5): 1026–34. doi:10.1016/j.mehy.2006.09.051. PMID 17145139.
- ^ DeLancey S (June 15, 2010). "FDA Cautions on Accurate Vitamin D Supplementation for Infants". Press Announcement. U.S. Food and Drug Administration.
- ^ Bischoff-Ferrari HA (2014). "Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes". Advances in Experimental Medicine and Biology (Review). 810: 500–25. PMID 25207384.
- ^ Harinarayan Vitamin D Status in India – Its Implications and Remedial Measures (2009) [cite http://www.japi.org/january_2009/R-1.html]a review of over 50 studies of 25(OH)D
- ^ Schoenmakers, Inez; Goldberg, Gail R.; Prentice, Ann (2008). "Abundant sunshine and vitamin D deficiency". British Journal of Nutrition. 99 (6): 1171–3. doi:10.1017/S0007114508898662. PMC 2758994. PMID 18234141.
- ^ Engelman, CD; Fingerlin, TE; Langefeld, CD; Hicks, PJ; Rich, SS; Wagenknecht, LE; Bowden, DW; Norris, JM (2008). "Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans". The Journal of Clinical Endocrinology and Metabolism. 93 (9): 3381–8. doi:10.1210/jc.2007-2702. PMC 2567851. PMID 18593774.
- ^ Creemers, PC; Du Toit, ED; Kriel, J (1995). "DBP (vitamin D binding protein) and BF (properdin factor B) allele distribution in Namibian San and Khoi and in other South African populations". Gene geography. 9 (3): 185–9. PMID 8740896.
- ^ Lips, P (2007). "Vitamin D status and nutrition in Europe and Asia". The Journal of Steroid Biochemistry and Molecular Biology. 103 (3–5): 620–5. doi:10.1016/j.jsbmb.2006.12.076. PMID 17287117.
- ^ Borges, CR; Rehder, DS; Jarvis, JW; Schaab, MR; Oran, PE; Nelson, RW (2010). "Full-length characterization of proteins in human populations". Clinical Chemistry. 56 (2): 202–11. doi:10.1373/clinchem.2009.134858. PMID 19926773.
- ^ Demer, LL; Tintut, Y (2008). "Vascular calcification: pathobiology of a multifaceted disease". Circulation. 117 (22): 2938–48. doi:10.1161/CIRCULATIONAHA.107.743161. PMID 18519861.
- ^ a b c d e f g h i j k l m Ross AC, Taylor CL, Yaktine AL, Del Valle HB (2011). Dietary Reference Intakes for Calcium and Vitamin D. Washington, D.C: National Academies Press. ISBN 0-309-16394-3.
- ^ Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, Lundqvist A, Jassal SK, Barrett-Connor E, Zhang C, Eaton CB, May HT, Anderson JL, Sesso HD; Song; Manson; Pilz; März; Michaëlsson; Lundqvist; Jassal; Barrett-Connor; Zhang; Eaton; May; Anderson; Sesso (November 2012). "Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies". Circ Cardiovasc Qual Outcomes. 5 (6): 819–29. doi:10.1161/CIRCOUTCOMES.112.967604. PMC 3510675. PMID 23149428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP; Tzoulaki; Zgaga; Ioannidis (April 1, 2014). "Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials". BMJ (Clinical research ed.). 348: g2035. doi:10.1136/bmj.g2035. PMC 3972415. PMID 24690624.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Autier P, Boniol M, Pizot C, Mullie P; Boniol; Pizot; Mullie (December 2013). "Vitamin D status and ill health: a systematic review". The Lancet Diabetes & Endocrinology. 2: 76–89. doi:10.1016/S2213-8587(13)70165-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Maxmen A (2011). "Nutrition advice: the vitamin D-lemma". Nature. 475 (7354): 23–5. doi:10.1038/475023a. PMID 21734684.
- ^ Bolland, Mark J; Grey, Andrew; Gamble, Greg D; Reid, Ian R (April 2014). "The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis". The Lancet Diabetes & Endocrinology. 2 (4): 307–320. doi:10.1016/S2213-8587(13)70212-2.
{{cite journal}}:|access-date=requires|url=(help) - ^ Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot Ld, Streppel M, Gardiner J, Ordóñez-Mena JM, Perna L, Wilsgaard T, Rathmann W, Feskens E, Kampman E, Siganos G, Njølstad I, Mathiesen EB, Kubínová R, Pająk A, Topor-Madry R, Tamosiunas A, Hughes M, Kee F, Bobak M, Trichopoulou A, Boffetta P, Brenner H, B.; Jorde, R.; Peasey, A.; Thorand; Jansen; Groot; Streppel; Gardiner; Ordóñez-Mena; Perna; Wilsgaard; Rathmann; Feskens; Kampman; Siganos; Njølstad; Mathiesen; Kubínová; Pająk; Topor-Madry; Tamosiunas; Hughes; Kee; Bobak; Trichopoulou; Boffetta; Brenner; Consortium on Health Ageing: Network of Cohorts in Europe the United States (June 17, 2014). "Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States". BMJ. 348 (jun17 16): g3656–g3656. doi:10.1136/bmj.g3656. PMC 4061380. PMID 24938302.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tuohimaa P (March 2009). "Vitamin D and aging". The Journal of Steroid Biochemistry and Molecular Biology. 114 (1–2): 78–84. doi:10.1016/j.jsbmb.2008.12.020. PMID 19444937.
- ^ Tuohimaa P, Keisala T, Minasyan A, Cachat J, Kalueff A; Keisala; Minasyan; Cachat; Kalueff (2009). "Vitamin D, nervous system and aging". Psychoneuroendocrinology. 34: S278–86. doi:10.1016/j.psyneuen.2009.07.003. PMID 19660871.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Manya H, Akasaka-Manya K, Endo T; Akasaka-Manya; Endo (July 2010). "Klotho protein deficiency and aging". Geriatr Gerontol Int. 10 (Suppl 1): S80–7. doi:10.1111/j.1447-0594.2010.00596.x. PMID 20590845.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Reid IR, Bolland MJ, Grey A; Bolland; Grey (January 11, 2014). "Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis". Lancet. 383 (9912): 146–55. doi:10.1016/s0140-6736(13)61647-5. PMID 24119980.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Avenell, A; Mak, JC; O'Connell, D (April 14, 2014). "Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men". The Cochrane database of systematic reviews. 4 (4): CD000227. doi:10.1002/14651858.CD000227.pub4. PMID 24729336.
- ^ Bischoff-Ferrari HA, Willett WC, Orav EJ, Oray EJ, Lips P, Meunier PJ, Lyons RA, Flicker L, Wark J, Jackson RD, Cauley JA, Meyer HE, Pfeifer M, Sanders KM, Stähelin HB, Theiler R, Dawson-Hughes B (July 2012). "A pooled analysis of vitamin D dose requirements for fracture prevention". N. Engl. J. Med. 367 (1): 40–9. doi:10.1056/NEJMoa1109617. PMID 22762317.
- ^ Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA (2011). "Vitamin D with or Without Calcium Supplementation for Prevention of Cancer and Fractures: An Updated Meta-analysis for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 155 (12): 827–38. doi:10.7326/0003-4819-155-12-201112200-00005. PMID 22184690.
- ^ Winzenberg T, Powell S, Shaw KA, Jones G (2011). "Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis". BMJ. 342: c7254. doi:10.1136/bmj.c7254. PMC 3026600. PMID 21266418.
- ^ Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V (August 2007). "Effectiveness and safety of vitamin D in relation to bone health". Evidence report/technology assessment (158): 1–235. PMC 4781354. PMID 18088161.
- ^ Bolland MJ, Grey A, Gamble GD, Reid IR (2014). "Vitamin D supplementation and falls: a trial sequential meta-analysis". Lancet Diabetes Endocrinol. 2 (7): 573–80. doi:10.1016/S2213-8587(14)70068-3. PMID 24768505.
- ^ Proposed Changes to the Nutrition Facts Label. FDA.gov (2016-05-20)
- ^ Shuler, F.D; Wingate, M.K; Moore, G.H; Giangarra, C (2012). "Sports health benefits of vitamin D". Sports Health. 4 (6): 496–501. doi:10.1177/1941738112461621. PMID 24179588.
- ^ Byers T (July 2010). "Anticancer vitamins du Jour--The ABCED's so far". Am. J. Epidemiol. (Review). 172 (1): 1–3. doi:10.1093/aje/kwq112. PMC 2892535. PMID 20562190.
- ^ Ma, Y; Zhang, P; Wang, F; Yang, J; Liu, Z; Qin, H (October 1, 2011). "Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies". Journal of Clinical Oncology. 29 (28): 3775–82. doi:10.1200/jco.2011.35.7566. PMID 21876081.
- ^ Feldman, D; Krishnan, AV; Swami, S; Giovannucci, E; Feldman, BJ (May 2014). "The role of vitamin D in reducing cancer risk and progression". Nature Reviews. Cancer. 14 (5): 342–57. doi:10.1038/nrc3691. PMID 24705652.
- ^ Chung, M; Lee, J; Terasawa, T; Lau, J; Trikalinos, TA (December 20, 2011). "Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 155 (12): 827–38. doi:10.7326/0003-4819-155-12-201112200-00005. PMID 22184690.
- ^ Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic M, Gluud C (January 10, 2014). "Vitamin D supplementation for prevention of mortality in adults". The Cochrane Database of Systematic Reviews. 1 (1): CD007470. doi:10.1002/14651858.cd007470.pub3. PMID 24414552.
- ^ Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G, Berruti A (2011). "Prognostic role of vitamin d status and efficacy of vitamin d supplementation in cancer patients: a systematic review". The Oncologist. 16 (9): 1215–27. doi:10.1634/theoncologist.2011-0098. PMC 3228169. PMID 21835895.
- ^ Li M, Chen P, Li J, Chu R, Xie D, Wang H (2014). "Review: the impacts of circulating 25-hydroxyvitamin D levels on cancer patient outcomes: a systematic review and meta-analysis". J Clin Endocrinol Metab. Online first (7): 2327–36. doi:10.1210/jc.2013-4320. PMID 24780061.
- ^ Beveridge, Louise A.; Struthers, Allan D.; Khan, Faisel; Jorde, Rolf; Scragg, Robert; Macdonald, Helen M.; Alvarez, Jessica A.; Boxer, Rebecca S.; Dalbeni, Andrea; Gepner, Adam D.; Isbel, Nicole M.; Larsen, Thomas; Nagpal, Jitender; Petchey, William G.; Stricker, Hans; Strobel, Franziska; Tangpricha, Vin; Toxqui, Laura; Vaquero, M. Pilar; Wamberg, Louise; Zittermann, Armin; Witham, Miles D. (March 16, 2015). "Effect of Vitamin D Supplementation on Blood Pressure". JAMA Internal Medicine. 175 (5): 745–54. doi:10.1001/jamainternmed.2015.0237. PMID 25775274.
- ^ Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, Li P, Davidson KW (2014). "Vitamin D Supplementation for Depressive Symptoms: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Psychosomatic Medicine. 76 (3): 190–6. doi:10.1097/psy.0000000000000044. PMC 4008710. PMID 24632894.
- ^ Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, Llewellyn DJ, Raina P (2012). "Vitamin D, cognition, and dementia: a systematic review and meta-analysis". Neurology. 79 (13): 1397–405. doi:10.1212/WNL.0b013e31826c197f. PMC 3448747. PMID 23008220.
- ^ Hewison M (2011). "Vitamin D and innate and adaptive immunity". Vitam. Horm. Vitamins & Hormones. 86: 23–62. doi:10.1016/B978-0-12-386960-9.00002-2. ISBN 9780123869609. PMID 21419266.
- ^ Beard JA, Bearden A, Striker R (March 2011). "Vitamin D and the anti-viral state". Journal of Clinical Virology. 50 (3): 194–200. doi:10.1016/j.jcv.2010.12.006. PMC 3308600. PMID 21242105.
- ^ Spector SA (February 2011). "Vitamin D and HIV: letting the sun shine in". Topics in antiviral medicine. 19 (1): 6–10. PMID 21852710.
- ^ Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E (2006). "Epidemic influenza and vitamin D". Epidemiology and Infection. 134 (6): 1129–40. doi:10.1017/S0950268806007175. PMC 2870528. PMID 16959053.
- ^ Nnoaham KE, Clarke A (February 2008). "Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis". International Journal of Epidemiology. 37 (1): 113–9. doi:10.1093/ije/dym247. PMID 18245055.
- ^ Luong Kv; Nguyen LT (June 2011). "Impact of vitamin D in the treatment of tuberculosis". The American journal of the medical sciences. 341 (6): 493–8. doi:10.1097/MAJ.0b013e3182070f47. PMID 21289501.
- ^ Yakoob, Mohammad Y; Salam, Rehana A; Khan, Farhan R; Bhutta, Zulfiqar A. "Vitamin D supplementation for preventing infections in children under five years of age". Cochrane Database of Systematic Reviews 2016. John Wiley & Sons, Ltd. doi:10.1002/14651858.cd008824.pub2/full (inactive November 18, 2016). Retrieved November 9, 2016.
{{cite journal}}: CS1 maint: DOI inactive as of November 2016 (link) - ^ Hart PH (2012). "Vitamin D supplementation, moderate sun exposure, and control of immune diseases". Discovery Medicine. 13 (73): 397–404. PMID 22742645.
- ^ Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC (January 2012). "Vitamin D and asthma". American Journal of Respiratory and Critical Care Medicine. 185 (2): 124–32. doi:10.1164/rccm.201108-1502CI. PMC 3297088. PMID 22016447.
- ^ Pierrot-Deseilligny C, Souberbielle JC (July 2010). "Is hypovitaminosis D one of the environmental risk factors for multiple sclerosis?". Brain : a journal of neurology. 133 (Pt 7): 1869–88. doi:10.1093/brain/awq147. PMID 20584945.
- ^ Pozuelo-Moyano B, Benito-León J, Mitchell AJ, Hernández-Gallego J (2013). "A systematic review of randomized, double-blind, placebo-controlled trials examining the clinical efficacy of vitamin D in multiple sclerosis". Neuroepidemiology (Systematic Review). 40 (3): 147–53. doi:10.1159/000345122. PMC 3649517. PMID 23257784.
the available evidence substantiates neither clinically significant benefit nor harm from vitamin D in the treatment of patients with MS
- ^ Pakpoor, J; Ramagopalan, S (December 13, 2014). "Evidence for an Association Between Vitamin D and Multiple Sclerosis". Current Topics in Behavioral Neurosciences. Current Topics in Behavioral Neurosciences. 26: 105–15. doi:10.1007/7854_2014_358. ISBN 978-3-319-25541-5. PMID 25502544.
- ^ a b Del Pinto, Rita; Pietropaoli, Davide; Chandar, Apoorva K.; Ferri, Claudio; Cominelli, Fabio (August 12, 2015). "Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis". Inflammatory Bowel Diseases. 21 (11): 2708–17. doi:10.1097/MIB.0000000000000546. ISSN 1536-4844. PMC 4615394. PMID 26348447.
- ^ a b Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM (2013). "Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies". BMJ. 346: f1169. doi:10.1136/bmj.f1169. PMID 23533188.
- ^ a b Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW (March 2012). "Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus". Nutrients. 4 (3): 208–30. doi:10.3390/nu4030208. PMC 3347028. PMID 22666547.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pathak, K.; Soares, M. J.; Calton, E. K.; Zhao, Y.; Hallett, J. (June 1, 2014). "Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials". Obesity Reviews. 15 (6): 528–537. doi:10.1111/obr.12162. ISSN 1467-789X. PMID 24528624.
- ^ Mallard, S. R.; Howe, A. S.; Houghton, L. A. (2016). "Vitamin D status and weight loss: A systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials". American Journal of Clinical Nutrition. 104 (4): 1151–1159. doi:10.3945/ajcn.116.136879. PMID 27604772.
- ^ Walter F. Boron (2003). "The Parathyroid Glands and Vitamin F". Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1094. ISBN 978-1-4160-2328-9.
- ^ Adams JS, Hewison M; Hewison (2010). "Update in Vitamin D". Journal of Clinical Endocrinology & Metabolism. 95 (2): 471–8. doi:10.1210/jc.2009-1773. PMC 2840860. PMID 20133466.
- ^ Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW; Levine; Bell; Mangelsdorf; Russell (May 2004). "Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase". Proc Natl Acad Sci U S A. 101 (20): 7711–7715. Bibcode:2004PNAS..101.7711C. doi:10.1073/pnas.0402490101. PMC 419671. PMID 15128933.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Laing CJ, Cooke NE (2004). "Section I: Ch. 8: Vitamin D Binding Protein". In Feldman D, Glorieux FH, Pike JW (eds.). Vitamin D. Vol. 1 (2 ed.). Academic Press. pp. 117–134. ISBN 0122526872.
- ^ Holick MF (1987). "Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables". Fed. Proc. 46 (5): 1876–82. PMID 3030826.
- ^ Deluca HF (January 2014). "History of the discovery of vitamin D and its active metabolites". Bonekey Rep. 3: 479. doi:10.1038/bonekey.2013.213. PMC 3899558. PMID 24466410.
- ^ a b Holick MF (March 2004). "Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis". The American Journal of Clinical Nutrition. 79 (3): 362–71. PMID 14985208.
- ^ Holick MF (2011). The Vitamin D Solution: A 3-Step Strategy to Cure Our Most Common Health Problems. New York: Plume. p. 27. ISBN 0-452-29688-9.
- ^ Crissey SD, Ange KD, Jacobsen KL, Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, Langman CB, Sadler W, Kahn S, Ward A; Ange; Jacobsen; Slifka; Bowen; Stacewicz-Sapuntzakis; Langman; Sadler; Kahn; Ward (2003). "Serum concentrations of lipids, vitamin D metabolites, retinol, retinyl esters, tocopherols and selected carotenoids in twelve captive wild felid species at four zoos". The Journal of Nutrition. 133 (1): 160–6. PMID 12514284.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hume EM, Lucas NS, Smith HH; Lucas; Smith (1927). "On the Absorption of vitamin D from the Skin". Biochemical Journal. 21 (2): 362–367. PMC 1251921. PMID 16743844.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ C. Claiborne Ray (May 17, 2005). "Sunshine Vitamin D". The New York Times. Archived from the original on February 21, 2013. Retrieved March 8, 2013.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Bolton J. "UV FAQs". Info. International Ultraviolet Association. Archived from the original on May 30, 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Holick MF (February 2002). "Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health". Current Opinion in Endocrinology, Diabetes and Obesity. 9 (1): 87–98. doi:10.1097/00060793-200202000-00011.
- ^ Holick MF (September 2002). "Sunlight and Vitamin D". Journal of General Internal Medicine. 17 (9): 733–735. doi:10.1046/j.1525-1497.2002.20731.x. PMC 1495109. PMID 12220371.
- ^ Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011). "8, Implications and Special Concerns". In Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds.). Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: National Academies Press. ISBN 0-309-16394-3. PMID 21796828.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ Yahav S, Buffenstein R; Buffenstein (1993). "Cholecalciferol supplementation alters gut function and improves digestibility in an underground inhabitant, the naked mole rat (Heterocephalus glaber), when fed on a carrot diet". The British journal of nutrition. 69 (1): 233–41. doi:10.1079/BJN19930025. PMID 8384476.
- ^ Stout SD, Agarwal SC (2003). Bone loss and osteoporosis: an anthropological perspective. New York: Kluwer Academic/Plenum Publishers. ISBN 0-306-47767-X.
- ^ Bouillon R, Van Cromphaut S, Carmeliet G; Van Cromphaut; Carmeliet (2003). "Intestinal calcium absorption: Molecular vitamin D mediated mechanisms". Journal of Cellular Biochemistry. 88 (2): 332–9. doi:10.1002/jcb.10360. PMID 12520535.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Bell TD, Demay MB, Burnett-Bowie SA; Demay; Burnett-Bowie (April 2010). "The biology and pathology of vitamin D control in bone". Journal of Cellular Biochemistry. 111 (1): 7–13. doi:10.1002/jcb.22661. PMC 4020510. PMID 20506379.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "An update on the association of vitamin D deficiency with common infectious diseases". Can J Physiol Pharmacol. 93 (5): 363–8. 2015. doi:10.1139/cjpp-2014-0352. PMID 25741906.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK; Stumpf; Stachowiak; Stachowiak (February 1996). "Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells". Molecular Brain Research. 36 (1): 193–6. doi:10.1016/0169-328X(95)00314-I. PMID 9011759.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sarkar FH, Li Y, Wang Z, Kong D; Li; Wang; Kong (2010). "The role of nutraceuticals in the regulation of Wnt and Hedgehog signaling in cancer". Cancer Metastasis Reviews. 29 (3): 383–64. doi:10.1007/s10555-010-9233-4. PMC 2974632. PMID 20711635.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Age-old children's disease back in force". Thestar.com. July 25, 2007. Retrieved August 24, 2010.
- ^ Elena Conis (July 24, 2006). "Fortified foods took out rickets". Los Angeles Times. Retrieved August 24, 2010.
- ^ McClean FC, Budy AM (January 28, 1964). "Vitamin A, Vitamin D, Cartilage, Bones, and Teeth". Vitamins and Hormones. Vol. 21. Academic Press. pp. 51–52. ISBN 978-0-12-709821-0.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ "History of Vitamin D". University of California at Riverside. 2011. Retrieved May 9, 2014.
- ^ "Adolf Windaus – Biography". Nobelprize.org. March 25, 2010. Retrieved March 25, 2010.
- ^ Rosenheim O, King H; King (1932). "The Ring-system of sterols and bile acids. Part II". J. Chem. Technol. Biotechnol. 51 (47): 954–7. doi:10.1002/jctb.5000514702.
- ^ Askew FA; Bourdillon RB; Bruce HM; Callow RK; St. L. Philpot J; Webster TA (1932). "Crystalline Vitamin D". Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. 109 (764): 488–506. doi:10.1098/rspb.1932.0008. JSTOR 81571.
- ^ Hirsch AL (2011). "Industrial aspects of vitamin D". In Feldman DJ, Pike JW, Adams JS (eds.). Vitamin D. London; Waltham, MA: Academic Press. p. 73. ISBN 978-0-12-387035-3.
- ^ Ziedonis AA, Mowery DC, Nelson RR, Bhaven NS (2004). Ivory tower and industrial innovation: university-industry technology transfer before and after the Bayh-Dole Act in the United States. Stanford, Calif: Stanford Business Books. pp. 39–40. ISBN 0-8047-4920-5.
- ^ Marshall J (2005). Elbridge A. Stuart Founder of the Carnation Company. Kessinger Publishing. p. 235. ISBN 978-1-4179-8883-9.
- ^ Holick MF, Schnoes HK, DeLuca HF (1971). "Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine". Proc. Natl. Acad. Sci. U.S.A. 68 (4): 803–4. Bibcode:1971PNAS...68..803H. doi:10.1073/pnas.68.4.803. PMC 389047. PMID 4323790.
- ^ Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popjak G (1971). "1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine". Science. 173 (3991): 51–4. Bibcode:1971Sci...173...51N. doi:10.1126/science.173.3991.51. PMID 4325863.
- ^ Holick MF, DeLuca HF, Avioli LV; Deluca; Avioli (1972). "Isolation and identification of 25-hydroxycholecalciferol from human plasma". Archives of Internal Medicine. 129 (1): 56–61. doi:10.1001/archinte.1972.00320010060005. PMID 4332591.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Eriksen EF; Mikkelsen; Poulsen; Hass; Overbeck; Thomsen; Charles; Eriksen (February 2000). "Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited". J. Intern. Med. 247 (2): 260–8. doi:10.1046/j.1365-2796.2000.00595.x. PMID 10692090.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Dietary Reference Intakes Tables [Health Canada, 2005]". Retrieved July 21, 2011.
- ^ Salleh, A. (June 12, 2012). "Vitamin D food fortification on the table". Australian Broadcasting Corporation.
- ^ "Nutrient reference values for Australia and New Zealand" (PDF). National Health and Medical Research Council. September 9, 2005. Retrieved December 11, 2010.
- ^ "Vitamin D and Calcium: Updated Dietary Reference Intakes". Nutrition and Healthy Eating. Health Canada. Retrieved June 13, 2012.
- ^ "Vitamins: what they do and where to find them (EUFIC)". European Food Information Council. December 10, 2010. Retrieved December 11, 2010.
Vitamin D
- ^ Vitamin-D-Bedarf bei fehlender endogener Synthese Deutsche Gesellschaft für Ernährung, January 2012
- ^ Pérez-López FR, Brincat M, Erel CT, Tremollieres F, Gambacciani M, Lambrinoudaki I, Moen MH, Schenck-Gustafsson K, Vujovic S, Rozenberg S, Rees M; Brincat; Erel; Tremollieres; Gambacciani; Lambrinoudaki; Moen; Schenck-Gustafsson; Vujovic; Rozenberg; Rees (January 2012). "EMAS position statement: Vitamin D and postmenopausal health". Maturitas. 71 (1): 83–8. doi:10.1016/j.maturitas.2011.11.002. PMID 22100145.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Vitamins and minerals – Vitamin D". National Health Service. February 18, 2015. Retrieved July 21, 2016.
- ^ "PHE publishes new advice on vitamin D". Public Health England. July 21, 2016. Retrieved July 21, 2016.
- ^ a b Heaney RP, Holick MF; Holick (2011). "Perspective: Why the IOM Recommendations for Vitamin D are Deficient". Journal of Bone and Mineral Research. 26 (3): 455–7. doi:10.1002/jbmr.328. PMID 21337617.
- ^ Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM (2011). "Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline". J Clin Endocrinol Metab. 96 (7): 1911–30. doi:10.1210/jc.2011-0385. PMID 21646368.
- ^ EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2012). "Scientific Opinion on the Tolerable Upper Intake Level of vitamin D". EFSA Journal. 10 (7): 2813. doi:10.2903/j.efsa.2012.2813.
- ^ European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) (2010). "Scientific opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 (2): 1468–85. doi:10.2903/j.efsa.2010.1468.
- ^ European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011). "Scientific opinion on the substantiation of a health claim related to vitamin D and risk of falling pursuant to Article 14 of Regulation (EC) No 1924/2006". EFSA Journal. 9 (9): 2382–2400. doi:10.2903/j.efsa.2011.2382.
- ^ "Guidance for Industry: Food Labeling: Health Claims; Calcium and Osteoporosis, and Calcium, Vitamin D, and Osteoporosis". US Food and Drug Administration. May 1, 2009.
- ^ "Health Canada Scientific Summary on the U. S. Health Claim Regarding Calcium and Osteoporosis". Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch Health Canada. May 1, 2000.
- ^ "Regulatory Systems of Health Claims in Japan" (PDF). Japan Consumer Affairs Agency, Food Labelling Division. June 1, 2011.
- ^ "Vitamin D". Nutrient Reference Values for Australia and New Zealand. Australian Ministry of Health. September 9, 2005.
- ^ Tripkovic L (2013). "Vitamin D2 vs. vitamin D3: Are they one and the same?". Nutrition Bulletin - Wiley Online Library. 38 (2): 243–248. doi:10.1111/nbu.12029. Retrieved April 27, 2015.
- ^ Alshahrani, Fahad; Aljohani, Naji (September 13, 2013). "Vitamin D: Deficiency, Sufficiency and Toxicity". Nutrients. 5 (9): 3605–3616. doi:10.3390/nu5093605. PMC 3798924. PMID 24067388. Retrieved April 27, 2015.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d e Keegan RJ, Lu Z, Bogusz JM, Williams JE, Holick MF; Lu; Bogusz; Williams; Holick (2013). "Photobiology of vitamin D in mushrooms and its bioavailability in humans". Dermato-Endocrinology. 5 (1): 165–76. doi:10.4161/derm.23321. PMC 3897585. PMID 24494050.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation". J Clin Endocrinol Metab. 98 (3): 973–9. 2013. doi:10.1210/jc.2012-2114. PMC 3590486. PMID 23386645.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Vitamin D bioavailability: state of the art". Crit Rev Food Sci Nutr. 55 (9): 1193–205. 2015. doi:10.1080/10408398.2012.688897. PMID 24915331.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b "Bringing Mushrooms Out of the Dark". MSNBC. April 18, 2006. Retrieved August 6, 2007.
- ^ Simon RR, Borzelleca JF, DeLuca HF, Weaver CM; Borzelleca; Deluca; Weaver (2013). "Safety assessment of the post-harvest treatment of button mushrooms (Agaricus bisporus) using ultraviolet light". Food and Chemical Toxicology. 56: 278–89. doi:10.1016/j.fct.2013.02.009. PMID 23485617.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "Search, National Nutrient Database for Standard Reference Release 27". US Department of Agriculture, Agricultural Research Service. 2014. Retrieved June 12, 2015.
- ^ Urbain P, Singler F, Ihorst G, Biesalski HK, Bertz H; Singler; Ihorst; Biesalski; Bertz (August 2011). "Bioavailability of vitamin D₂ from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial". Eur J Clin Nutr. 65 (8): 965–71. doi:10.1038/ejcn.2011.53. PMID 21540874.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Koyyalamudi SR, Jeong SC, Song CH, Cho KY, Pang G; Jeong; Song; Cho; Pang (2009). "Vitamin D2 formation and bioavailability from Agaricus bisporus button mushrooms treated with ultraviolet irradiation". J Agric Food Chem. 57 (8): 3351–5. doi:10.1021/jf803908q. PMID 19281276.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Duke J. "Dr. Duke's Phytochemical and Ethnobotanical Databases". U.S. Agricultural Research Service.
- ^ DRI, Dietary reference intakes: for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, D.C: National Academy Press. 1997. p. 250. ISBN 0-309-06350-7.
- ^ Wang T, Bengtsson G, Kärnefelt I, Björn LO; Bengtsson; Kärnefelt; Björn (September 2001). "Provitamins and vitamins D₂and D₃in Cladina spp. over a latitudinal gradient: possible correlation with UV levels". J. Photochem. Photobiol. B, Biol. 62 (1–2): 118–22. doi:10.1016/S1011-1344(01)00160-9. PMID 11693362.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Holick MF (2005). "The Vitamin D Epidemic and its Health Consequences" (PDF). Journal of Nutrition. 135 (11): 2739S–48S. PMID 16251641.
- ^ Takeuchi A, Okano T, Sayamoto M, Sawamura S, Kobayashi T, Motosugi M, Yamakawa T; Okano; Sayamoto; Sawamura; Kobayashi; Motosugi; Yamakawa (1986). "Tissue distribution of 7-dehydrocholesterol, vitamin D3 and 25-hydroxyvitamin D3 in several species of fishes". Journal of nutritional science and vitaminology. 32 (1): 13–22. doi:10.3177/jnsv.32.13. PMID 3012050.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Jakobsen, Jette; Knuthsen, Pia (2014). "Stability of vitamin D in foodstuffs during cooking". Food Chemistry. 148: 170–175. doi:10.1016/j.foodchem.2013.10.043. PMID 24262542.
- ^ a b c Hoel, David G.; Berwick, Marianne; de Gruijl, Frank R.; Holick, Michael F. (2016). "The risks and benefits of sun exposure 2016". Dermato-Endocrinology. 8 (1): e1248325. doi:10.1080/19381980.2016.1248325. PMID 27942349.
- ^ Costello RB (2009). "Vitamin D and health in the 21st century: federal initiatives to advance research". Am J Med Sci. 338 (1): 34–9. doi:10.1097/MAJ.0b013e3181aaee78. PMID 19593101.
- ^ "How is vitamin D being studied now in clinical cancer research?". Bethesda, MD: National Cancer Institute, US National Institutes of Health. October 21, 2013.
- ^ "About the National Health and Nutrition Examination Survey". Atlanta, GA: National Center for Health Statistics, US Centers for Disease Control. November 6, 2015.
- ^ "Prevalence and correlates of vitamin D deficiency in US adults". Nutr Res. 31 (1): 48–54. 2011. doi:10.1016/j.nutres.2010.12.001. PMID 21310306.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "ODS Vitamin D Initiative". Bethesda, MD: Office of Dietary Supplements, US National Institutes of Health. 2014.
- ^ "Vitamin D: An overview of vitamin D status and intake in Europe". Nutr Bull. 39 (4): 322–350. 2014. PMID 25635171.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ Funderburk, L. K.; Daigle, K; Arsenault, J. E. (2015). "Vitamin D status among overweight and obese soldiers". Military Medicine. 180 (2): 237–40. doi:10.7205/MILMED-D-14-00041. PMID 25643393.
- ^ Olson, M. L.; Maalouf, N. M.; Oden, J. D.; White, P. C.; Hutchison, M. R. (2012). "Vitamin D deficiency in obese children and its relationship to glucose homeostasis". The Journal of Clinical Endocrinology & Metabolism. 97 (1): 279–85. doi:10.1210/jc.2011-1507. PMC 3251943. PMID 22072738.
- ^ Turer, C. B.; Lin, H; Flores, G (2013). "Prevalence of vitamin D deficiency among overweight and obese US children" (PDF). Pediatrics. 131 (1): e152–61. doi:10.1542/peds.2012-1711. PMID 23266927.
- ^ Pyrżak, B; Witkowska-Sędek, E; Krajewska, M; Demkow, U; Kucharska, A. M. (2015). "Metabolic and immunological consequences of vitamin D deficiency in obese children". Adv Exp Med Biol. 840: 13-19. doi:10.1007/5584_2014_81. PMID 25315624.
Further reading
- NIH Vitamin D Fact Sheet for Health Professionals from the U.S. National Institutes of Health
- Disagreement among experts about the correct vitamin D dose. (Nature News, July 6, 2011)
External links
- Vitamin D in children from the Royal National Orthopaedic Hospital Trust
