Nicotinamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌnaɪəˈsɪnəmaɪd/, /ˌnɪkəˈtɪnəmaɪd/ |
| Other names | NAM, 3-pyridinecarboxamide niacinamide nicotinic acid amide vitamin PP nicotinic amide vitamin B3 |
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Routes of administration | oral, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.467 |
| Chemical and physical data | |
| Formula | C6H6N2O |
| Molar mass | 122.127 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3 g/cm3 [1] |
| Melting point | 129.5 °C (265.1 °F) |
| Boiling point | 334 °C (633 °F) |
| |
| |
Niacinamide or nicotinamide is a form of vitamin B3 found in food and used as a dietary supplement and medication.[2][3][4] As a supplement, it is used orally (swallowed by mouth) to prevent and treat pellagra (niacin deficiency).[3] While nicotinic acid (niacin) may be used for this purpose, niacinamide has the benefit of not causing skin flushing.[3] As a cream, it is used to treat acne, and has been observed in clinical studies to improve the appearance of aging skin by reducing hyperpigmentation and redness.[4][5] It is a water-soluble vitamin. Niacinamide is the supplement name, while nicotinamide is the scientific name.
Side effects are minimal.[6][7] At high doses, liver problems may occur.[6] Normal amounts are safe for use during pregnancy.[8] Niacinamide is in the vitamin B family of medications, specifically the vitamin B3 complex.[9][10] It is an amide of nicotinic acid.[6] Foods that contain niacinamide include yeast, meat, milk, and green vegetables.[11]
Niacinamide was discovered between 1935 and 1937.[12][13] It is on the World Health Organization's List of Essential Medicines.[14][15] Niacinamide is available as a generic medication and over the counter.[9] Commercially, niacinamide is made from either nicotinic acid (niacin) or nicotinonitrile.[13][16] In some countries, grains have niacinamide added to them.[13]
Medical uses[edit]
Niacin deficiency[edit]
Niacinamide is the preferred treatment for pellagra, caused by niacin deficiency.[3]
Acne[edit]
Niacinamide cream is used as a treatment for acne.[4] It has anti-inflammatory actions, which may benefit people with inflammatory skin conditions.[17]
Niacinamide increases the biosynthesis of ceramides in human keratinocytes in vitro and improves the epidermal permeability barrier in vivo.[18] The application of 2% topical niacinamide for 2 and 4 weeks has been found to be effective in lowering the sebum excretion rate.[19] Niacinamide has been shown to prevent Cutibacterium acnes-induced activation of toll-like receptor 2, which ultimately results in the down-regulation of pro-inflammatory interleukin-8 production.[20]
Skin cancer[edit]
Niacinamide at doses of 500 to 1000 mg a day decreases the risk of skin cancers, other than melanoma, in those at high risk.[21]
Side effects[edit]
Niacinamide has minimal side effects.[6][7] At very high doses above 3g/ day acute liver toxicity has been documented in at least one case.[6] Normal doses are safe during pregnancy.[8]
Chemistry[edit]
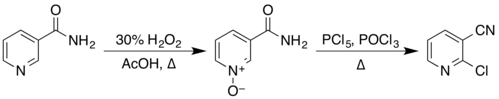
The structure of nicotinamide consists of a pyridine ring to which a primary amide group is attached in the meta position. It is an amide of nicotinic acid.[6] As an aromatic compound, it undergoes electrophilic substitution reactions and transformations of its two functional groups. Examples of these reactions reported in Organic Syntheses include the preparation of 2-chloronicotinonitrile by a two-step process via the N-oxide,[22][23]
from nicotinonitrile by reaction with phosphorus pentoxide,[24] and from 3-aminopyridine by reaction with a solution of sodium hypobromite, prepared in situ from bromine and sodium hydroxide.[25]

Industrial production[edit]
The hydrolysis of nicotinonitrile is catalysed by the enzyme nitrile hydratase from Rhodococcus rhodochrous J1,[26][27][16] producing 3500 tons per annum of nicotinamide for use in animal feed.[28] The enzyme allows for a more selective synthesis as further hydrolysis of the amide to nicotinic acid is avoided.[29][30] Nicotinamide can also be made from nicotinic acid. According to Ullmann's Encyclopedia of Industrial Chemistry, worldwide 31,000 tons of nicotinamide were sold in 2014.[13]
Biochemistry[edit]

Nicotinamide, as a part of the cofactor nicotinamide adenine dinucleotide (NADH / NAD+) is crucial to life. In cells, nicotinamide is incorporated into NAD+ and nicotinamide adenine dinucleotide phosphate (NADP+). NAD+ and NADP+ are cofactors in a wide variety of enzymatic oxidation-reduction reactions, most notably glycolysis, the citric acid cycle, and the electron transport chain.[31] If humans ingest nicotinamide, it will likely undergo a series of reactions that transform it into NAD, which can then undergo a transformation to form NADP+. This method of creation of NAD+ is called a salvage pathway. However, the human body can produce NAD+ from the amino acid tryptophan and niacin without our ingestion of nicotinamide.[32]
NAD+ acts as an electron carrier that mediates the interconversion of energy between nutrients and the cell's energy currency, adenosine triphosphate (ATP). In oxidation-reduction reactions, the active part of the cofactor is the nicotinamide. In NAD+, the nitrogen in the aromatic nicotinamide ring is covalently bonded to adenine dinucleotide. The formal charge on the nitrogen is stabilized by the shared electrons of the other carbon atoms in the aromatic ring. When a hydride atom is added onto NAD+ to form NADH, the molecule loses its aromaticity, and therefore a good amount of stability. This higher energy product later releases its energy with the release of a hydride, and in the case of the electron transport chain, it assists in forming adenosine triphosphate.[33]
When one mole of NADH is oxidized, 158.2 kJ of energy will be released.[33]
Biological role[edit]
Nicotinamide occurs as a component of a variety of biological systems, including within the vitamin B family and specifically the vitamin B3 complex.[9][10] It is also a critically important part of the structures of NADH and NAD+, where the N-substituted aromatic ring in the oxidised NAD+ form undergoes reduction with hydride attack to form NADH.[31] The NADPH/NADP+ structures have the same ring, and are involved in similar biochemical reactions.
Nicotinamide can be methylated in the liver to biologically active 1-Methylnicotinamide when there are sufficient methyl donors.
Food sources[edit]
Niacinamide occurs in trace amounts mainly in meat, fish, nuts, and mushrooms, as well as to a lesser extent in some vegetables.[34] It is commonly added to cereals and other foods. Many multivitamins contain 20–30 mg of vitamin B3 and it is also available in higher doses.[35]
Compendial status[edit]
Research[edit]
A 2015 trial found niacinamide to reduce the rate of new nonmelanoma skin cancers and actinic keratoses in a group of people at high risk for the conditions.[38]
Niacinamide has been investigated for many additional disorders, including treatment of bullous pemphigoid nonmelanoma skin cancers.[39]
Niacinamide may be beneficial in treating psoriasis.[40]
There is tentative evidence for a potential role of niacinamide in treating acne, rosacea, autoimmune blistering disorders, ageing skin, and atopic dermatitis.[39] Niacinamide also inhibits poly(ADP-ribose) polymerases (PARP-1), enzymes involved in the rejoining of DNA strand breaks induced by radiation or chemotherapy.[41] ARCON (accelerated radiotherapy plus carbogen inhalation and nicotinamide) has been studied in cancer.[42]
Research has suggested niacinamide may play a role in the treatment of HIV.[43]
References[edit]
- ^ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ Bender DA (2003). Nutritional Biochemistry of the Vitamins. Cambridge University Press. p. 203. ISBN 978-1-139-43773-8. Archived from the original on 30 December 2016.
- ^ a b c d World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 496, 500. hdl:10665/44053. ISBN 978-92-4-154765-9.
- ^ a b c British National Formulary: BNF 69 (69th ed.). British Medical Association. 2015. p. 822. ISBN 978-0-85711-156-2.
- ^ Bissett DL, Oblong JE, Berge CA (July 2005). "Niacinamide: A B vitamin that improves aging facial skin appearance". Dermatologic Surgery. 31 (7 Pt 2): 860–5, discussion 865. doi:10.1111/j.1524-4725.2005.31732. PMID 16029679.
- ^ a b c d e f Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, Bingley PJ, et al. (November 2000). "Safety of high-dose nicotinamide: a review" (PDF). Diabetologia. 43 (11): 1337–45. doi:10.1007/s001250051536. PMID 11126400. S2CID 24763480. Archived (PDF) from the original on 22 September 2017. Retrieved 20 April 2018.
- ^ a b MacKay D, Hathcock J, Guarneri E (June 2012). "Niacin: chemical forms, bioavailability, and health effects". Nutrition Reviews. 70 (6): 357–66. doi:10.1111/j.1753-4887.2012.00479.x. PMID 22646128.
- ^ a b "Niacinamide Use During Pregnancy". Drugs.com. Archived from the original on 30 December 2016. Retrieved 29 December 2016.
- ^ a b c "Niacinamide: Indications, Side Effects, Warnings". Drugs.com. 6 June 2017. Archived from the original on 5 August 2017. Retrieved 30 June 2017.
- ^ a b Krutmann J, Humbert P (2010). Nutrition for Healthy Skin: Strategies for Clinical and Cosmetic Practice. Springer Science & Business Media. p. 153. ISBN 978-3-642-12264-4. Archived from the original on 10 April 2017.
- ^ Burtis CA, Ashwood ER, Bruns DE (2012). Tietz Textbook of Clinical Chemistry and Molecular Diagnostics (5th ed.). Elsevier Health Sciences. p. 934. ISBN 978-1-4557-5942-2. Archived from the original on 30 December 2016.
- ^ Sneader W (2005). Drug Discovery: A History. John Wiley & Sons. p. 231. ISBN 978-0-470-01552-0. Archived from the original on 30 December 2016.
- ^ a b c d Blum R (2015). "Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide)". Vitamins, 11. Niacin (Nicotinic Acid, Nicotinamide. Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). Weinheim: Wiley-VCH. pp. 1–9. doi:10.1002/14356007.o27_o14.pub2. ISBN 978-3-527-30385-4.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ a b Schmidberger JW, Hepworth LJ, Green AP, Flitsch SL (2015). "Enzymatic Synthesis of Amides". In Faber K, Fessner WD, Turner NJ (eds.). Biocatalysis in Organic Synthesis 1. Science of Synthesis. Georg Thieme Verlag. pp. 329–372. ISBN 978-3-13-176611-3. Archived from the original on 5 November 2017.
- ^ Niren NM (January 2006). "Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: a review". Cutis. 77 (1 Suppl): 11–6. PMID 16871774.
- ^ Tanno O, Ota Y, Kitamura N, Katsube T, Inoue S (September 2000). "Nicotinamide increases biosynthesis of ceramides as well as other stratum corneum lipids to improve the epidermal permeability barrier". The British Journal of Dermatology. 143 (3): 524–31. doi:10.1111/j.1365-2133.2000.03705.x. PMID 10971324. S2CID 21874670.
- ^ Draelos ZD, Matsubara A, Smiles K (June 2006). "The effect of 2% niacinamide on facial sebum production". Journal of Cosmetic and Laser Therapy. 8 (2): 96–101. doi:10.1080/14764170600717704. PMID 16766489. S2CID 36713665.
- ^ Kim J, Ochoa MT, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. (August 2002). "Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses". Journal of Immunology. 169 (3): 1535–41. doi:10.4049/jimmunol.169.3.1535. PMC 4636337. PMID 12133981.
- ^ Snaidr VA, Damian DL, Halliday GM (February 2019). "Nicotinamide for photoprotection and skin cancer chemoprevention: A review of efficacy and safety". Experimental Dermatology. 28 (Suppl 1): 15–22. doi:10.1111/exd.13819. PMID 30698874.
- ^ Taylor EC, Crovetti AJ (1957). "Nicotinamide-1-oxide". Organic Syntheses. 37: 63. doi:10.15227/orgsyn.037.0063; Collected Volumes, vol. 4, p. 704.
- ^ Taylor EC, Crovetti AJ (1957). "2-Chloronicitinonitrile". Organic Syntheses. 37: 12. doi:10.15227/orgsyn.037.0012; Collected Volumes, vol. 4, p. 166.
- ^ Teague PC, Short WA (1953). "Nicotinonitrile". Organic Syntheses. 33: 52. doi:10.15227/orgsyn.033.0052; Collected Volumes, vol. 4, p. 706.
- ^ Allen CF, Wolf CN (1950). "3-Aminopyridine". Organic Syntheses. 30: 3. doi:10.15227/orgsyn.030.0003; Collected Volumes, vol. 4, p. 45.
- ^ Nagasawa T, Mathew CD, Mauger J, Yamada H (July 1988). "Nitrile Hydratase-Catalyzed Production of Nicotinamide from 3-Cyanopyridine in Rhodococcus rhodochrous J1". Applied and Environmental Microbiology. 54 (7): 1766–1769. Bibcode:1988ApEnM..54.1766N. doi:10.1128/AEM.54.7.1766-1769.1988. PMC 202743. PMID 16347686.
- ^ Hilterhaus L, Liese A (2007). "Building Blocks". In Ulber R, Sell D (eds.). White Biotechnology. Advances in Biochemical Engineering / Biotechnology. Vol. 105. Springer Science & Business Media. pp. 133–173. doi:10.1007/10_033. ISBN 978-3-540-45695-7. PMID 17408083. S2CID 34552222. Archived from the original on 5 November 2017.
- ^ Asano Y (2015). "Hydrolysis of Nitriles to Amides". In Faber K, Fessner WD, Turner NJ (eds.). Biocatalysis in Organic Synthesis 1. Science of Synthesis. Georg Thieme Verlag. pp. 255–276. ISBN 978-3-13-176611-3. Archived from the original on 5 November 2017.
- ^ Petersen M, Kiener A (1999). "Biocatalysis". Green Chem. 1 (2): 99–106. doi:10.1039/A809538H.
- ^ Servi S, Tessaro D, Hollmann F (2015). "Historical Perspectives: Paving the Way for the Future". In Faber NJ, Fessner WD, Turner K (eds.). Biocatalysis in Organic Synthesis 1. Science of Synthesis. Georg Thieme Verlag. pp. 1–39. ISBN 978-3-13-176611-3. Archived from the original on 5 November 2017.
- ^ a b Belenky P, Bogan KL, Brenner C (January 2007). "NAD+ metabolism in health and disease" (PDF). Trends in Biochemical Sciences. 32 (1): 12–9. doi:10.1016/j.tibs.2006.11.006. PMID 17161604. Archived (PDF) from the original on 27 September 2007.
- ^ Williams AC, Cartwright LS, Ramsden DB (March 2005). "Parkinson's disease: the first common neurological disease due to auto-intoxication?". QJM. 98 (3): 215–26. doi:10.1093/qjmed/hci027. PMID 15728403.
- ^ a b Casiday R, Herman C, Frey R (5 September 2008). "Energy for the Body: Oxidative Phosphorylation". www.chemistry.wustl.edu. Department of Chemistry, Washington University in St. Louis. Archived from the original on 22 November 2016. Retrieved 14 March 2017.
- ^ Rolfe HM (December 2014). "A review of nicotinamide: treatment of skin diseases and potential side effects". Journal of Cosmetic Dermatology. 13 (4): 324–8. doi:10.1111/jocd.12119. PMID 25399625. S2CID 28160151.
- ^ Ranaweera A (2017). "Nicotinamide". DermNet New Zealand (www.dermnetnz.org). DermNet New Zealand Trust. Archived from the original on 25 March 2017. Retrieved 30 June 2017.
- ^ British Pharmacopoeia Commission Secretariat (2009). Index, BP 2009 (PDF). Archived from the original (PDF) on 22 July 2011. Retrieved 4 February 2010.
- ^ Japanese Pharmacopoeia (PDF) (15th ed.). 2006. Archived from the original (PDF) on 22 July 2011. Retrieved 4 February 2010.
- ^ Minocha R, Damian DL, Halliday GM (January 2018). "Melanoma and nonmelanoma skin cancer chemoprevention: A role for nicotinamide?". Photodermatology, Photoimmunology & Photomedicine. 34 (1): 5–12. doi:10.1111/phpp.12328. PMID 28681504.
- ^ a b Chen AC, Damian DL (August 2014). "Nicotinamide and the skin". The Australasian Journal of Dermatology. 55 (3): 169–75. doi:10.1111/ajd.12163. PMID 24635573. S2CID 45745255.
- ^ Namazi MR (August 2003). "Nicotinamide: a potential addition to the anti-psoriatic weaponry". FASEB Journal. 17 (11): 1377–9. doi:10.1096/fj.03-0002hyp. PMID 12890690. S2CID 39752891.
- ^ "Definition of niacinamide". NCI Drug Dictionary. National Cancer Institute. 2 February 2011. Archived from the original on 28 April 2015. Retrieved 30 June 2017.
- ^ Kaanders JH, Bussink J, van der Kogel AJ (December 2002). "ARCON: a novel biology-based approach in radiotherapy". The Lancet. Oncology. 3 (12): 728–37. doi:10.1016/s1470-2045(02)00929-4. PMID 12473514.
- ^ Mandavilli A (7 July 2020). "Patient Is Reported Free of H.I.V., but Scientists Urge Caution". The New York Times. Archived from the original on 23 September 2020. Retrieved 22 September 2020.