Aromatic compound
An aromatic hydrocarbon (abbreviated as AH) or arene [1] (or sometimes aryl hydrocarbon)[2] is a hydrocarbon with a conjugated cyclic molecular structure that is much more stable than the hypothetical localized structure. The term 'aromatic' was assigned before the physical mechanism determining aromaticity was discovered, and was derived from the fact that many of the compounds have a sweet scent. The configuration of six carbon atoms in aromatic compounds is known as a benzene ring, after the simplest possible such hydrocarbon, benzene. Aromatic hydrocarbons can be monocyclic or polycyclic.
Some non-benzene-based compounds called heteroarenes, which follow Hückel's rule, are also aromatic compounds. In these compounds, at least one carbon atom is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes an oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom.[3]
Benzene ring model

Benzene, C6H6, is the simplest AH and was recognized as the first aromatic hydrocarbon, with the nature of its bonding first being recognized by Friedrich August Kekulé von Stradonitz in the 19th century. Each carbon atom in the hexagonal cycle has four electrons to share. One goes to the hydrogen atom, and one each to the two neighboring carbons. This leaves one to share with one of its two neighboring carbon atoms, which is why the benzene molecule is drawn with alternating single and double bonds around the hexagon.
The structure is also illustrated as a circle around the inside of the ring to show six electrons floating around in delocalized molecular orbitals the size of the ring itself. This also represents the equivalent nature of the six carbon-carbon bonds all of bond order ~1.5. This equivalency is well explained by resonance forms. The electrons are visualized as floating above and below the ring with the electromagnetic fields they generate acting to keep the ring flat.
General properties:
- Display aromaticity.
- The carbon-hydrogen ratio is high.
- They burn with a sooty yellow flame because of the high carbon-hydrogen ratio.
- They undergo electrophilic substitution reactions and nucleophilic aromatic substitutions.
The circle symbol for aromaticity was introduced by Sir Robert Robinson in 1925 and popularized starting in 1959 by the Morrison & Boyd textbook on organic chemistry. The proper use of the symbol is debated, it is used to describe any cyclic pi system in some publications, or only those pi systems that obey Hückel's rule on others. Jensen [4] argues that in line with Robinson's original proposal, the use of the circle symbol should be limited to monocyclic 6 pi-electron systems. In this way the circle symbol for a 6c–6e bond can be compared to the Y symbol for a 3c–2e bond.
Arene synthesis
A reaction that forms an arene compound from an unsaturated or partially unsaturated cyclic precursor is simply called an aromatization. Many laboratory methods exist for the organic synthesis of arenes from non-arene precursors. Many methods rely on cycloaddition reactions. Alkyne trimerization describes the [2+2+2] cyclization of three alkynes, in the Dötz reaction an alkyne, carbon monoxide and a chromium carbene complex are the reactants.Diels-Alder reactions of alkynes with pyrone or cyclopentadienone with expulsion of carbon dioxide or carbon monoxide also form arene compounds. In Bergman cyclization the reactants are an enyne plus a hydrogen donor.
Another set of methods is the aromatization of cyclohexanes and other aliphatic rings: reagents are catalysts used in hydrogenation such as platinum, palladium and nickel (reverse hydrogenation), quinones and the elements sulfur and selenium.[5]
Arene reactions
Arenes are reactants in many organic reactions.
Aromatic substitution
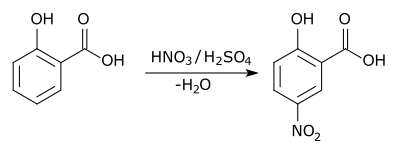
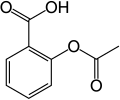
In aromatic substitution one substituent on the arene ring, usually hydrogen, is replaced by another substituent. The two main types are electrophilic aromatic substitution when the active reagent is an electrophile and nucleophilic aromatic substitution when the reagent is a nucleophile. In radical-nucleophilic aromatic substitution the active reagent is a radical. An example is the nitration of salicylic acid [6]:
Coupling reactions
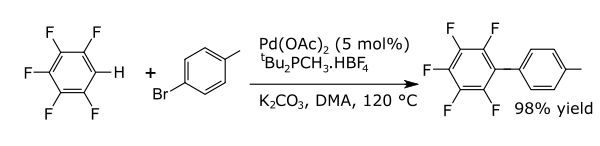
In coupling reactions a metal catalyses a coupling between two formal radical fragments. Commons coupling reactions with arenes result in the formation of new carbon-carbon bonds e.g. alkylarenes, vinyl arenes, biraryls, new carbon-nitrogen bonds (anilines) or new carbon-oxygen bonds (aryloxy compounds). An example is the direct arylation of perfluorobenzenes [7]
Hydrogenation
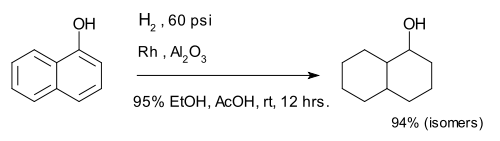
Hydrogenation of arenes create saturated rings. The compound 1-naphthol is completely reduced to a mixture of decalin-ol isomers.[8]
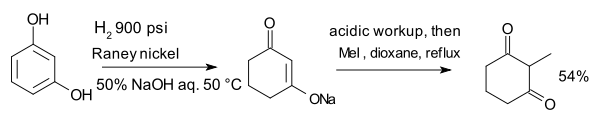
The compound resorcinol, hydrogenated with Raney nickel in presence of aqueous sodium hydroxide forms an enolate which is alkylated with methyl iodide to 2-methyl-1,3-cyclohexandione:[9]
Cycloadditions
Cycloaddition reaction are not common. Unusual thermal Diels-Alder reactivity of arenes can be found in the Wagner-Jauregg reaction. Other photochemical cycloaddition reactions with alkenes occur through excimers.
Benzene and derivatives of benzene
Benzene derivatives have from one to six substituents attached to the central benzene core. Examples of benzene compounds with just one substituent are phenol, which carries a hydroxyl group and toluene with a methyl group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ortho, meta, and para are devised. For example, three isomers exist for cresol because the methyl group and the hydroxyl group can be placed next to each other (ortho), one position removed from each other (meta), or two positions removed from each other (para). Xylenol has two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist.
- Representative arene compounds
The arene ring has an ability to stabilize charges. This is seen in, for example, phenol (C6H5-OH), which is acidic at the hydroxyl (OH), since a charge on this oxygen (alkoxide -O–) is partially delocalized into the benzene ring.
Polyaromatic hydrocarbons

Some important arenes are the polyaromatic hydrocarbons (PAH); they are also called polycyclic aromatic hydrocarbons and polynuclear aromatic hydrocarbons. They are composed of more than one aromatic ring. The simplest PAHs are benzocyclopropene (C7H6), benzocyclopropane (C7H8), benzocyclobutadiene (C8H6), and benzocyclobutene (C8H8). A simple synthesis of benzocyclopropene is published [1].
Common examples are naphthalene with two fused rings, anthracene with three, tetracene with four, and pentacene with five linearly fused rings. Phenanthrene and triphenylene are examples of non-linear connections. More exotic examples are helicenes and corannulene.
These compounds are among the most widespread organic pollutants, remaining on beaches and marine environmentals for a long time after an oil spill. Recent investigations have concluced that their toxicity is up to 100 times worse than first assumed.[10]
See also
External links
- Carcinogenic FAC list in Portable Document Format.
- Toxicological profiles of PAH.
- LIST of PAH.
- Abiogenic Gas Debate 11:2002 (EXPLORER)
References
- ^ Definition IUPAC Gold Book Link
- ^ Mechanisms of Activation of the Aryl Hydrocarbon Receptor by Maria Backlund, Institute of Environmental Medicine, Karolinska Institutet
- ^ HighBeam Encyclopedia: aromatic compound
- ^ The Origin of the Circle Symbol for Aromaticity by William B. Jensen 424 Journal of Chemical Education Vol. 86 No. 4 April 2009
- ^ Jerry March Advanced Organic Chemistry 3Ed., ISBN 0-471-85472-7
- ^ Template:Cite DOI
- ^ Template:Cite DOI
- ^ Organic Syntheses, Coll. Vol. 6, p.371 (1988); Vol. 51, p.103 (1971). http://orgsynth.org/orgsyn/pdfs/CV6P0371.pdf
- ^ Organic Syntheses, Coll. Vol. 5, p.743 (1973); Vol. 41, p.56 (1961). http://orgsynth.org/orgsyn/pdfs/CV5P0567.pdf
- ^ "Sound battles back, but threats linger". NOAA Fisheries. Retrieved 2008-02-02.