Hydroxymethylglutaryl-CoA synthase
| 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 (soluble) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | HMGCS1 | ||||||
| Alt. symbols | HMGCS | ||||||
| NCBI gene | 3157 | ||||||
| HGNC | 5007 | ||||||
| OMIM | 142940 | ||||||
| RefSeq | NM_002130 | ||||||
| UniProt | Q01581 | ||||||
| Other data | |||||||
| EC number | 2.3.3.10 | ||||||
| Locus | Chr. 5 p14-p13 | ||||||
| |||||||
| 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 (mitochondrial) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | HMGCS2 | ||||||
| NCBI gene | 3158 | ||||||
| HGNC | 5008 | ||||||
| OMIM | 600234 | ||||||
| RefSeq | NM_005518 | ||||||
| UniProt | P54868 | ||||||
| Other data | |||||||
| Locus | Chr. 1 p13-p12 | ||||||
| |||||||
| Hydroxymethylglutaryl-coenzyme A synthase N terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 staphylococcus aureus 3-hydroxy-3-methylglutaryl-coa synthase | |||||||||
| Identifiers | |||||||||
| Symbol | HMG_CoA_synt_N | ||||||||
| Pfam | PF01154 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR013528 | ||||||||
| PROSITE | PDOC00942 | ||||||||
| |||||||||
| Hydroxymethylglutaryl-coenzyme A synthase C terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 staphylococcus aureus 3-hydroxy-3-methylglutaryl-coa synthase | |||||||||
| Identifiers | |||||||||
| Symbol | HMG_CoA_synt_C | ||||||||
| Pfam | PF08540 | ||||||||
| Pfam clan | CL0046 | ||||||||
| InterPro | IPR013746 | ||||||||
| PROSITE | PDOC00942 | ||||||||
| |||||||||
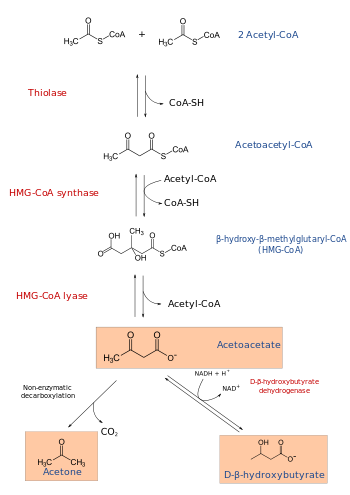
In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.

The 3 substrates of this enzyme are acetyl-CoA, H2O, and acetoacetyl-CoA, whereas its two products are (S)-3-hydroxy-3-methylglutaryl-CoA and CoA.
In humans, the protein is encoded by the HMGCS1 gene on chromosome 5.
Classification[edit]
This enzyme belongs to the family of transferases, specifically those acyltransferases that convert acyl groups into alkyl groups on transfer.
Nomenclature[edit]
The systematic name of this enzyme class is acetyl-CoA:acetoacetyl-CoA C-acetyltransferase (thioester-hydrolysing, carboxymethyl-forming). Other names in common use include (S)-3-hydroxy-3-methylglutaryl-CoA acetoacetyl-CoA-lyase, (CoA-acetylating), 3-hydroxy-3-methylglutaryl CoA synthetase, 3-hydroxy-3-methylglutaryl coenzyme A synthase, 3-hydroxy-3-methylglutaryl coenzyme A synthetase, 3-hydroxy-3-methylglutaryl-CoA synthase, 3-hydroxy-3-methylglutaryl-coenzyme A synthase, beta-hydroxy-beta-methylglutaryl-CoA synthase, HMG-CoA synthase, acetoacetyl coenzyme A transacetase, hydroxymethylglutaryl coenzyme A synthase, and hydroxymethylglutaryl coenzyme A-condensing enzyme.
Mechanism[edit]
HMG-CoA synthase contains an important catalytic cysteine residue that acts as a nucleophile in the first step of the reaction: the acetylation of the enzyme by acetyl-CoA (its first substrate) to produce an acetyl-enzyme thioester, releasing the reduced coenzyme A. The subsequent nucleophilic attack on acetoacetyl-CoA (its second substrate) leads to the formation of HMG-CoA.[1]
Biological role[edit]
This enzyme participates in 3 metabolic pathways: synthesis and degradation of ketone bodies, valine, leucine and isoleucine degradation, and butanoate metabolism.
Species distribution[edit]
HMG-CoA synthase occurs in eukaryotes, archaea, and certain bacteria.[2]
Eukaryotes[edit]
In vertebrates, there are two different isozymes of the enzyme (cytosolic and mitochondrial); in humans the cytosolic form has only 60.6% amino acid identity with the mitochondrial form of the enzyme. HMG-CoA is also found in other eukaryotes such as insects, plants, and fungi.[3]
Cytosolic[edit]
The cytosolic form is the starting point of the mevalonate pathway, which leads to cholesterol and other sterolic and isoprenoid compounds.

Mitochondrial[edit]
The mitochondrial form is responsible for the biosynthesis of ketone bodies. The gene for the mitochondrial form of the enzyme has three sterol regulatory elements in the 5' flanking region.[4] These elements are responsible for decreased transcription of the message responsible for enzyme synthesis when dietary cholesterol is high in animals: the same is observed for 3-hydroxy-3-methylglutaryl-CoA and the low density lipoprotein receptor.
Bacteria[edit]
In bacteria, isoprenoid precursors are generally synthesised via an alternative, non-mevalonate pathway, however a number of Gram-positive pathogens utilise a mevalonate pathway involving HMG-CoA synthase that is parallel to that found in eukaryotes.[5][6]
Structural studies[edit]
As of late 2007, 4 structures have been solved for this class of enzymes, with PDB accession codes 1XPK, 1XPL, 1XPM, and 2P8U.
External links[edit]
- HMG-CoA+synthase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
References[edit]
- ^ Theisen MJ, Misra I, Saadat D, Campobasso N, Miziorko HM, Harrison DH (November 2004). "3-hydroxy-3-methylglutaryl-CoA synthase intermediate complex observed in "real-time"". Proc. Natl. Acad. Sci. U.S.A. 101 (47): 16442–7. doi:10.1073/pnas.0405809101. PMC 534525. PMID 15498869.
- ^ Bahnson BJ (November 2004). "An atomic-resolution mechanism of 3-hydroxy-3-methylglutaryl-CoA synthase". Proc. Natl. Acad. Sci. U.S.A. 101 (47): 16399–400. Bibcode:2004PNAS..10116399B. doi:10.1073/pnas.0407418101. PMC 534547. PMID 15546978.
- ^ Bearfield JC, Keeling CI, Young S, Blomquist GJ, Tittiger C (April 2006). "Isolation, endocrine regulation and mRNA distribution of the 3-hydroxy-3-methylglutaryl coenzyme A synthase (HMG-S) gene from the pine engraver, Ips pini (Coleoptera: Scolytidae)". Insect Molecular Biology. 15 (2): 187–95. doi:10.1111/j.1365-2583.2006.00627.x. PMID 16640729. S2CID 46317830.
- ^ Goldstein J.L., Brown M.S. (1990) Regulation of the mevalonate pathway. Nature 343, 425-430
- ^ Steussy CN, Robison AD, Tetrick AM, Knight JT, Rodwell VW, Stauffacher CV, Sutherlin AL (December 2006). "A structural limitation on enzyme activity: the case of HMG-CoA synthase". Biochemistry. 45 (48): 14407–14. doi:10.1021/bi061505q. PMID 17128980.
- ^ Steussy CN, Vartia AA, Burgner JW, Sutherlin A, Rodwell VW, Stauffacher CV (November 2005). "X-ray crystal structures of HMG-CoA synthase from Enterococcus faecalis and a complex with its second substrate/inhibitor acetoacetyl-CoA". Biochemistry. 44 (43): 14256–67. doi:10.1021/bi051487x. PMID 16245942.
- RUDNEY H (1957). "The biosynthesis of beta-hydroxy-beta-methylglutaric acid". J. Biol. Chem. 227 (1): 363–77. doi:10.1016/S0021-9258(18)70822-3. PMID 13449080.

