Vitamin D
| Vitamin D | |
|---|---|
| Drug class | |
 Cholecalciferol (D3) | |
| Class identifiers | |
| Synonyms | Calciferols |
| Use | Rickets, osteoporosis, osteomalacia, vitamin D deficiency |
| ATC code | A11CC |
| Biological target | vitamin D receptor |
| Clinical data | |
| Drugs.com | MedFacts Natural Products |
| External links | |
| MeSH | D014807 |
| Legal status | |
| In Wikidata | |
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions.[1][2] In humans, the most significant compounds within this group are vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol).[2][3]
The primary natural source of vitamin D is the synthesis of cholecalciferol in the lower layers of the skin’s epidermis, triggered by a photochemical reaction with ultraviolet B (UV-B) radiation from sunlight or UV-B lamps.[1] Cholecalciferol and ergocalciferol can also be obtained through diet and supplements.[1][2] Foods such as the flesh of fatty fish are good sources of vitamin D, though there are few other foods where it naturally appears in significant amounts.[2][4] In the U.S. and other countries, cow's milk and plant-based milk substitutes are fortified with vitamin D, as are many breakfast cereals.[1] Mushrooms exposed to ultraviolet light also provide useful amounts of vitamin D2.[2][5] Dietary recommendations typically assume that all of a person's vitamin D is taken by mouth, given the variability in sunlight exposure among the population and uncertainties regarding safe levels of sunlight exposure, particularly due to the associated risk of skin cancer.[2]
Vitamin D obtained from the diet or synthesised in the skin is biologically inactive. It becomes active by two enzymatic hydroxylation steps, the first occurring in the liver and the second in the kidneys.[1][3] Since most mammals can synthesise sufficient vitamin D with adequate sunlight exposure, it is technically not essential in the diet and thus not a true vitamin. Instead it functions as a hormone; the activation of the vitamin D pro-hormone produces calcitriol, the active form. Calcitriol then exerts its effects via the vitamin D receptor, a nuclear receptor found in various tissues throughout the body.[6]
Cholecalciferol is converted in the liver to calcifediol (also known as calcidiol or 25-hydroxycholecalciferol), while ergocalciferol is converted to ercalcidiol (25-hydroxyergocalciferol).[1] These two vitamin D metabolites, collectively referred to as 25-hydroxyvitamin D or 25(OH)D, are measured in serum to assess a person's vitamin D status.[7][8] Calcifediol is further hydroxylated by the kidneys and certain immune cells to form calcitriol (1,25-dihydroxycholecalciferol), the biologically active form of vitamin D.[9][10] Calcitriol circulates in the blood as a hormone, playing a major role in regulating calcium and phosphate concentrations, as well as promoting bone health and bone remodeling.[1] Additionally, calcitriol has other effects, including influencing cell differentiation, neuromuscular and immune functions, and reducing inflammation.[2]
Vitamin D has a significant role in calcium homeostasis and metabolism.[1] Its discovery was due to effort to identify the dietary deficiency in children with rickets, the childhood form of osteomalacia.[11] Vitamin D supplements are commonly used to treat or to prevent osteomalacia and rickets.[1] The evidence for other health benefits of vitamin D supplementation in individuals who are already vitamin D sufficient is inconsistent.[2] The effect of vitamin D supplementation on morbidity and mortality is also unclear, with one meta-analysis finding a small decrease in mortality in elderly people.[12] Except for the prevention of rickets and osteomalacia in high-risk groups, any benefit of vitamin D supplements to musculoskeletal or general health may be small and in some cases, may have adverse effects on health.[13][14][15]
Types
[edit]| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | Mixture of molecular compounds of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) | 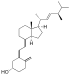
|
| Vitamin D3 | cholecalciferol
(made from 7-dehydrocholesterol in the skin). |

|
| Vitamin D4 | 22-dihydroergocalciferol | 
|
| Vitamin D5 | sitocalciferol
(made from 7-dehydrositosterol) |

|
Several forms (vitamers) of vitamin D exist, with the two major forms being vitamin D2 or ergocalciferol, and vitamin D3 or cholecalciferol.[1] The term 'vitamin D' refers to either D2 or D3, or both, and is known collectively as calciferol.[16]
Vitamin D2 was chemically characterized in 1931. In 1935, the chemical structure of vitamin D3 was defined and shown to result from the ultraviolet irradiation of 7-dehydrocholesterol. Although a chemical nomenclature for vitamin D forms was recommended in 1981,[17] alternative names remain commonly used.[3]
Chemically, the various forms of vitamin D are secosteroids, meaning that one of the bonds in the steroid rings is broken.[18] The structural difference between vitamin D2 and vitamin D3 lies in the side chain: vitamin D2 has a double bond between carbons 22 and 23, and a methyl group on carbon 24.[3] Numerous vitamin D analogues have also been synthesized.[3]
Biology
[edit]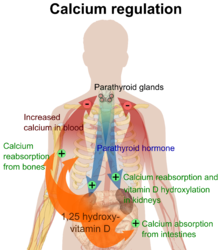
The active vitamin D metabolite, calcitriol, exerts its biological effects by binding to the vitamin D receptor (VDR), which is primarily located in the nuclei of target cells.[1][18] When calcitriol binds to the VDR, it enables the receptor to act as a transcription factor, modulating the gene expression of transport proteins involved in calcium absorption in the intestine, such as TRPV6 and calbindin.[20] The VDR is part of the nuclear receptor superfamily of steroid hormone receptors, which are hormone-dependent regulators of gene expression. These receptors are expressed in cells across most organs.
Activation of VDR in the intestine, bone, kidney, and parathyroid gland cells plays a crucial role in maintaining calcium and phosphorus levels in the blood, a process that is assisted by parathyroid hormone and calcitonin, thereby supporting bone health.[1][21]
One of the most important functions of vitamin D is to maintain skeletal calcium balance by promoting calcium absorption in the intestines, promoting bone resorption by increasing osteoclast numbers, maintaining calcium and phosphate levels necessary for bone formation, and facilitating the proper function of parathyroid hormone to sustain serum calcium levels.[1] Vitamin D deficiency can lead to decreased bone mineral density, increasing the risk of osteoporosis and bone fractures due to its impact on mineral metabolism.[1][22] Consequently, vitamin D is also important for bone remodeling, acting as a potent stimulator of bone resorption.[22]
The VDR also regulates cell proliferation and differentiation. Additionally, vitamin D influences the immune system, with VDRs being expressed in several white blood cells, including monocytes and activated T and B cells.[23] In vitro studies indicate that vitamin D increases the expression of the tyrosine hydroxylase gene in adrenal medullary cells and affects the synthesis of neurotrophic factors, nitric oxide synthase, and glutathione, which may control the body's response and adaption to stress.[24]
VDR expression decreases with age.[1]
Deficiency
[edit]A diet insufficient in vitamin D, combined with inadequate sunlight exposure, can lead to vitamin D deficiency, which is defined as a blood 25-hydroxyvitamin D or 25(OH)D level below 12 ng/mL (30 nmol/liter). Vitamin D insufficiency is characterized by a blood 25(OH)D level between 12–20 ng/mL (30–50 nmol/liter).[2][25] It is estimated that one billion adults worldwide are either vitamin D insufficient or deficient, including those in developed countries across Europe.[26] Severe vitamin D deficiency in children, although rare in the developed world, can cause a softening and weakening of growing bones, leading to a condition known as rickets.[27]
Vitamin D deficiency is prevalent globally, particularly among the elderly, and remains common in both children and adults.[28][29][30] This deficiency impairs bone mineralization and causes bone damage, leading to bone-softening diseases such as rickets in children and osteomalacia in adults.[31] Low blood calcifediol (25-hydroxyvitamin D3) levels can result from limited sun exposure.[32] When vitamin D levels are deficient, the total absorption of dietary calcium can decrease from the normal range of 60–80% to 15%.[21]
Dark-skinned individuals living in temperate climates are more likely to have low vitamin D levels.[33][34][35] This is because melanin in the skin, which hinders vitamin D synthesis, makes dark-skinned individuals less efficient at producing vitamin D.[36] In the U.S., vitamin D deficiency is particularly common among Hispanic and African-American populations.[25]
Health effects
[edit]Supplementation with vitamin D is a reliable method for preventing or treating rickets.[1] On the other hand, the effects of vitamin D supplementation on non-skeletal health are uncertain.[37][38] A review did not find any effect from supplementation on the rates of non-skeletal disease, other than a tentative decrease in mortality in the elderly.[39] Vitamin D supplements do not alter the outcomes for myocardial infarction, stroke or cerebrovascular disease, cancer, bone fractures or knee osteoarthritis.[14][40]
A US Institute of Medicine (IOM) report states: "Outcomes related to cancer, cardiovascular disease and hypertension, and diabetes and metabolic syndrome, falls and physical performance, immune functioning and autoimmune disorders, infections, neuropsychological functioning, and preeclampsia could not be linked reliably with intake of either calcium or vitamin D, and were often conflicting."[41]: 5 Some researchers claim the IOM was too definitive in its recommendations and made a mathematical mistake when calculating the blood level of vitamin D associated with bone health.[42] Members of the IOM panel maintain that they used a "standard procedure for dietary recommendations" and that the report is solidly based on the data.[42]
Mortality, all-causes
[edit]Vitamin D3 supplementation has been tentatively found to lead to a reduced risk of death in the elderly,[12][39] but the effect has not been deemed pronounced, or certain enough, to make taking supplements recommendable.[14] Other forms (vitamin D2, alfacalcidol, and calcitriol) do not appear to have any beneficial effects with regard to the risk of death.[12] High blood levels appear to be associated with a lower risk of death, but it is unclear if supplementation can result in this benefit.[43] Both an excess and a deficiency in vitamin D appear to cause abnormal functioning and premature aging.[44][45] The relationship between serum calcifediol concentrations and all-cause mortality is "U-shaped": mortality is elevated at high and low calcifediol levels, relative to moderate levels.[41] Harm from vitamin D appears to occur at a lower vitamin D level in the dark skinned Canadian and United States populations which have been studied than in the light skinned Canadian and United States populations which have been studied. Whether this is so with dark skinned populations in other parts of the world is unknown.[41]: 435
Bone health
[edit]Rickets
[edit]Rickets, a childhood disease, is characterized by impeded growth and soft, weak, deformed long bones that bend and bow under their weight as children start to walk. Maternal vitamin D deficiency can cause fetal bone defects from before birth and impairment of bone quality after birth.[46][47] Rickets typically appear between 3 and 18 months of age.[48] This condition can be caused by vitamin D, calcium or phosphorus deficiency.[49] Vitamin D deficiency remains the main cause of rickets among young infants in most countries because breast milk is low in vitamin D, and darker skin, social customs and climatic conditions can contribute to inadequate sun exposure.[citation needed] A post-weaning Western omnivore diet characterized by high intakes of meat, fish, eggs and vitamin D fortified milk is protective, whereas low intakes of those foods and high cereal/grain intake contributes to risk.[21][50][51][52] For young children with rickets, supplementation with vitamin D plus calcium was superior to the vitamin alone for bone healing.[53]
Osteomalacia and osteoporosis
[edit]Characteristics of osteomalacia are softening of the bones, leading to bending of the spine, bone fragility, and increased risk for fractures.[1] Osteomalacia is usually present when 25-hydroxyvitamin D levels are less than about 10 ng/mL.[54] Osteomalacia progress to osteoporosis, a condition of reduced bone mineral density with increased bone fragility and risk of bone fractures. Osteoporosis can be a long-term effect of calcium and/or vitamin D insufficiency, the latter contributing by reducing calcium absorption.[2] In the absence of confirmed vitamin D deficiency there is no evidence that vitamin D supplementation without concomitant calcium slows or stops the progression of osteomalacia to osteoporosis.[13] For older people with osteoporosis, taking vitamin D with calcium may help prevent hip fractures, but it also slightly increases the risk of stomach and kidney problems.[55][56] The reduced rick for fractures is not seen in healthier, community-dwelling elderly.[57][58] Low serum vitamin D levels have been associated with falls,[59] but taking extra vitamin D does not appear to reduce that risk.[60]
Athletes who are vitamin D deficient are at an increased risk of stress fractures and/or major breaks, particularly those engaging in contact sports. Incremental decreases in risk are observed with rising serum 25(OH)D concentrations plateauing at 50 ng/mL with no additional benefits seen in levels beyond this point.[61]
Cancer
[edit]Potential associations have been found between low vitamin D levels and the risk of developing several types of cancer.[62][63][64] Meta-analyses of observational studies have found reduced risk of cancer incidence related to vitamin D intake and 25(OH)D levels, particularly for colorectal cancer, although the strength of the associations was classified as weak.[64][65] Vitamin D receptor and SNAI2 are found to be involved in the metastastic process of osteosarcoma.[66] While randomized controlled trials have not confirmed that vitamin D supplements reduce the risk of cancer incidence, the relative risk of cancer deaths was lower by up to 16% in several meta-analyses.[67][65]
Low levels of 25-hydroxyvitamin D, a routinely used marker for vitamin D, have been suggested as a contributing factor in increasing the risk the development and progression of various types of cancer, including melanoma. Vitamin D requires activation by cytochrome P450 (CYP) enzymes to become active and bind to the VDR. Specifically, CYP27A1, CYP27B1, and CYP2R1 are involved in the activation of vitamin D, while CYP24A1 and CYP3A4 are responsible for the degradation of the active vitamin D. CYP24A1, the primary catabolic enzyme of calcitriol, is overexpressed in melanoma tissues and cells. This overexpression could lead to lower levels of active vitamin D in tissues, potentially promoting the development and progression of melanoma. Several drug classes and natural health products can modulate vitamin D-related CYP enzymes, potentially causing lower levels of vitamin D and its active metabolites in tissues, suggesting that maintaining adequate vitamin D levels, that is, avoiding vitamin D deficiency, either through dietary supplements or by modulating CYP metabolism, could be beneficial in decreasing the risk of melanoma development.[62]
Cardiovascular disease
[edit]Vitamin D supplementation is not associated with a reduced risk of stroke, cerebrovascular disease, myocardial infarction, or ischemic heart disease.[14][68][69] Supplementation does not lower blood pressure in the general population.[70][71][72]
Immune system
[edit]Infectious diseases
[edit]In general, vitamin D functions to activate the innate and dampen the adaptive immune systems with antibacterial, antiviral and anti-inflammatory effects.[73][74] Low serum levels of vitamin D appear to be a risk factor for tuberculosis.[75] However, supplementation trials showed no benefit.[76][77] Vitamin D supplementation at low doses may slightly decrease the overall risk of acute respiratory tract infections.[78] The benefits were found in children and adolescents, and were not confirmed with higher doses.[78]
Inflammatory bowel disease
[edit]Vitamin D deficiency has been linked to the severity of inflammatory bowel disease (IBD).[79] However, whether vitamin D deficiency causes IBD or is a consequence of the disease is not clear.[80] Supplementation leads to improvements in scores for clinical inflammatory bowel disease activity and biochemical markers and[81][80] less frequent relapse of symptoms in IBD.[80]
Other conditions
[edit]Chronic obstructive pulmonary disease
[edit]Vitamin D supplementation substantially reduced the rate of moderate or severe exacerbations of chronic obstructive pulmonary disease (COPD).[82]
Asthma
[edit]Vitamin D supplementation does not help prevent asthma attacks or alleviate symptoms.[83]
Diabetes
[edit]A meta-analysis reported that vitamin D supplementation significantly reduced the risk of type 2 diabetes for non-obese people with prediabetes.[84] Another meta-analysis reported that vitamin D supplementation significantly improved glycemic control [homeostatic model assessment-insulin resistance (HOMA-IR)], hemoglobin A1C (HbA1C), and fasting blood glucose (FBG) in individuals with type 2 diabetes.[85] In prospective studies, high versus low level of vitamin D was respectively associated with significant decrease in risk of type 2 diabetes, combined type 2 diabetes and prediabetes, and prediabetes.[86] A 2011 Cochrane systematic review examined one study that showed vitamin D together with insulin maintained levels of fasting C-peptide after 12 months better than insulin alone. However, it is important to highlight that the studies available to be included in this review presented considerable flaws in quality and design.[87]
Attention deficit hyperactivity disorder (ADHD)
[edit]A meta-analysis of observational studies showed that children with ADHD have lower vitamin D levels, and that there was a small association between low vitamin D levels at the time of birth and later development of ADHD.[88] Several small, randomized controlled trials of vitamin D supplementation indicated improved ADHD symptoms such as impulsivity and hyperactivity.[89]
Depression
[edit]Clinical trials of vitamin D supplementation for depressive symptoms have generally been of low quality and show no overall effect, although subgroup analysis showed supplementation for participants with clinically significant depressive symptoms or depressive disorder had a moderate effect.[90]
Cognition and dementia
[edit]A systematic review of clinical studies found an association between low vitamin D levels with cognitive impairment and a higher risk of developing Alzheimer's disease. However, lower vitamin D concentrations are also associated with poor nutrition and spending less time outdoors. Therefore, alternative explanations for the increase in cognitive impairment exist and hence a direct causal relationship between vitamin D levels and cognition could not be established.[91]
Schizophrenia
[edit]People diagnosed with schizophrenia tend to have lower serum vitamin D concentration compared to those without the condition. This may be a consequence of the disease rather than a cause, due, for example, to low dietary vitamin D and less time spent exposed to sunlight.[92][93] Results from supplementation trials have been inconclusive.[92]
Pregnancy
[edit]Low levels of vitamin D in pregnancy are associated with gestational diabetes, pre-eclampsia, and small (for gestational age) infants.[94] Although taking vitamin D supplements during pregnancy raises blood levels of vitamin D in the mother at term,[95] the full extent of benefits for the mother or baby is unclear.[94][95][96][97] Pregnant women often do not take the recommended amount of vitamin D,[98] however, the benefits and risk of vitamin D supplementation during pregnancy have not been well studied.[97]
Obesity
[edit]Obesity increases the risk of having low serum vitamin D. Supplementation does not lead to weight loss, but weight loss increases serum vitamin D. The theory is that fatty tissue sequesters vitamin D.[99]
Uterine fibroids
[edit]There is evidence that pathogenesis of uterine fibroids is associated with low serum vitamin D and that supplementation reduces size of fibroids.[100][101]
Allowed health claims
[edit]Governmental regulatory agencies stipulate for the food and dietary supplement industries certain health claims as allowable as statements on packaging.
Europe: European Food Safety Authority (EFSA)
- normal function of the immune system[102]
- normal inflammatory response[102]
- normal muscle function[102]
- reduced risk of falling in people over age 60[103]
US: Food and Drug Administration (FDA)
- "Adequate calcium and vitamin D, as part of a well balanced diet, along with physical activity, may reduce the risk of osteoporosis."[104]
Canada: Health Canada
- "Adequate calcium and regular exercise may help to achieve strong bones in children and adolescents and may reduce the risk of osteoporosis in older adults. An adequate intake of vitamin D is also necessary."[105]
Japan: Foods with Nutrient Function Claims (FNFC)
- "Vitamin D is a nutrient which promotes the absorption of calcium in the gut intestine and aids in the development of bone."[106]
Australia-New Zealand.[107]
Dietary intake
[edit]| United Kingdom | ||
| Age group | Intake (μg/day) | Maximum intake (μg/day)[108] |
|---|---|---|
| Breast-fed infants 0–12 months | 8.5 – 10 | 25 |
| Formula-fed infants (<500 mL/d) | 10 | 25 |
| Children 1 – 10 years | 10 | 50 |
| Children >10 and adults | 10 | 100 |
| United States | ||
| Age group | RDA (IU/day) | (μg/day)[41] |
| Infants 0–6 months | 400* | 10 |
| Infants 6–12 months | 400* | 10 |
| 1–70 years | 600 | 15 |
| Adults > 70 years | 800 | 20 |
| Pregnant/Lactating | 600 | 15 |
| Age group | Tolerable upper intake level (IU/day) | (μg/day) |
| Infants 0–6 months | 1,000 | 25 |
| Infants 6–12 months | 1,500 | 37.5 |
| 1–3 years | 2,500 | 62.5 |
| 4–8 years | 3,000 | 75 |
| 9+ years | 4,000 | 100 |
| Pregnant/lactating | 4,000 | 100[41] |
| Canada | ||
| Age group | RDA (IU)[109] | Tolerable upper intake (IU)[109] |
| Infants 0–6 months | 400* | 1,000 |
| Infants 7–12 months | 400* | 1,500 |
| Children 1–3 years | 600 | 2,500 |
| Children 4–8 years | 600 | 3,000 |
| Children and adults 9–70 years | 600 | 4,000 |
| Adults > 70 years | 800 | 4,000 |
| Pregnancy & lactation | 600 | 4,000 |
| Australia and New Zealand | ||
| Age group | Adequate Intake (μg)[107] | Upper Level of Intake (μg)[107] |
| Infants 0–12 months | 5* | 25 |
| Children 1–18 years | 5* | 80 |
| Adults 19–50 years | 5* | 80 |
| Adults 51–70 years | 10* | 80 |
| Adults > 70 years | 15* | 80 |
| European Food Safety Authority | ||
| Age group | Adequate Intake (μg)[110] | Tolerable upper limit (μg)[111] |
| Infants 0–12 months | 10 | 25 |
| Children 1–10 years | 15 | 50 |
| Children 11–17 years | 15 | 100 |
| Adults | 15 | 100 |
| Pregnancy & Lactation | 15 | 100 |
| * Adequate intake, no RDA/RDI yet established | ||
Recommended levels
[edit]Various government institutions have proposed different recommendations for the amount of daily intake of vitamin D. These vary according to precise definition, age, pregnancy or lactation, and the extent assumptions are made regarding skin synthesis of vitamin D.[2][41][107][108][109][110] Conversion: 1 μg (microgram) = 40 IU (international unit).[108]
United Kingdom
[edit]The UK National Health Service (NHS) recommends that people at risk of vitamin D deficiency, breast-fed babies, formula-fed babies taking less than 500 ml/day, and children aged 6 months to 4 years, should take daily vitamin D supplements throughout the year to ensure sufficient intake.[108] This includes people with limited skin synthesis of vitamin D, who are not often outdoors, are frail, housebound, living in a care home, or usually wearing clothes that cover up most of the skin, or with dark skin, such as having an African, African-Caribbean or south Asian background. Other people may be able to make adequate vitamin D from sunlight exposure from April to September. The NHS and Public Health England recommend that everyone, including those who are pregnant and breastfeeding, consider taking a daily supplement containing 10 μg (400 IU) of vitamin D during autumn and winter because of inadequate sunlight for vitamin D synthesis.[112]
United States
[edit]The dietary reference intake for vitamin D issued in 2010 by the Institute of Medicine (IoM) (renamed National Academy of Medicine in 2015), superseded previous recommendations which were expressed in terms of adequate intake. The recommendations were formed assuming the individual has no skin synthesis of vitamin D because of inadequate sun exposure. The reference intake for vitamin D refers to total intake from food, beverages and supplements, and assumes that calcium requirements are being met.[41]: 5 The tolerable upper intake level (UL)[113] is defined as "the highest average daily intake of a nutrient that is likely to pose no risk of adverse health effects for nearly all persons in the general population."[41]: 403 Although ULs are believed to be safe, information on the long-term effects is incomplete and these levels of intake are not recommended for long-term consumption.[41]: 403 : 433
For US food and dietary supplement labeling purposes, the amount in a serving is expressed as a percent of Daily Value (%DV). For vitamin D labeling purposes, 100% of the daily value was 400 IU (10 μg), but in May 2016, it was revised to 800 IU (20 μg) to bring it into agreement with the recommended dietary allowance (RDA).[114][115] A table of the old and new adult daily values is provided at Reference Daily Intake.
Canada
[edit]Health Canada published recommended dietary intakes (DRIs) and tolerable upper intake levels (ULs) for vitamin D based on the jointly commissioned and funded Institute of Medicine 2010 report.[41][109]
Australia and New Zealand
[edit]Australia and New Zealand published nutrient reference values including guidelines for dietary vitamin D intake in 2006.[107] About a third of Australians have vitamin D deficiency.[116][117]
European Union
[edit]The European Food Safety Authority (EFSA) in 2016[110] reviewed the current evidence, finding the relationship between serum 25(OH)D concentration and musculoskeletal health outcomes is widely variable. They considered that average requirements and population reference intakes values for vitamin D cannot be derived, and that a serum 25(OH)D concentration of 50 nmol/L was a suitable target value. For all people over the age of 1, including women who are pregnant or lactating, they set an adequate intake of 15 μg/day (600 IU).[110]
The EFSA reviewed safe levels of intake in 2012,[111] setting the tolerable upper limit for adults at 100 μg/day (4000 IU), a similar conclusion as the IOM.
The Swedish National Food Agency recommends a daily intake of 10 μg (400 IU) of vitamin D3 for children and adults up to 75 years, and 20 μg (800 IU) for adults 75 and older.[118]
Non-government organisations in Europe have made their own recommendations. The German Society for Nutrition recommends 20 μg.[119] The European Menopause and Andropause Society recommends postmenopausal women consume 15 μg (600 IU) until age 70, and 20 μg (800 IU) from age 71. This dose should be increased to 100 μg (4,000 IU) in some patients with very low vitamin D status or in case of co-morbid conditions.[120]
Sources
[edit]In general, vitamin D3 is found in animal source foods, particularly fish, meat, offal, egg and dairy.[121] It is commonly added as a fortification in manufactured foods.[41] Vitamin D2 is found in fungi and is produced by ultraviolet irradiation of ergosterol.[122] The vitamin D2 content in mushrooms and Cladina arbuscula, a lichen, increases with exposure to ultraviolet light,[123][124] and is stimulated by industrial ultraviolet lamps for fortification.[122] The United States Department of Agriculture reports D2 and D3 content combined in one value.
Natural sources
[edit]| Animal sources | |||
| Source[125] | IU/g | Irregular | |
|---|---|---|---|
| Cooked egg yolk | 0.7 | 44 IU for a 61g egg | |
| Beef liver, cooked, braised | 0.5 | ||
| Fish liver oils, such as cod liver oil | 100 | 450 IU per teaspoon (4.5 g) | |
| Fatty fish species | |||
| Salmon, pink, cooked, dry heat | 5.2 | ||
| Mackerel, Pacific and jack, mixed species, cooked, dry heat | 4.6 | ||
| Tuna, canned in oil | 2.7 | ||
| Sardines, canned in oil, drained | 1.9 | ||
| Fungal sources | |||
| Source | μg/g | IU/g | |
|---|---|---|---|
| Cladonia arbuscula (lichen), thalli, dry[123] | vitamin D3 | 0.67–2.04 | 27–82 |
| vitamin D2 | 0.22–0.55 | 8.8–22 | |
| Agaricus bisporus (common mushroom): D2 + D3 | |||
| Portobello | Raw | 0.003 | 0.1 |
| Exposed to ultraviolet light | 0.11 | 4.46 | |
| Crimini | Raw | 0.001 | 0.03 |
| Exposed to ultraviolet light | 0.32 | 12.8 | |
Food fortification
[edit]Manufactured foods fortified with vitamin D include some fruit juices and fruit juice drinks, meal replacement energy bars, soy protein-based beverages, certain cheese and cheese products, flour products, infant formulas, many breakfast cereals, and milk.[126][127]
In 2016 in the United States, the Food and Drug Administration (FDA) amended food additive regulations for milk fortification,[128] stating that vitamin D3 levels not exceed 42 IU vitamin D per 100 g (400 IU per US quart) of dairy milk, 84 IU of vitamin D2 per 100 g (800 IU per quart) of plant milks, and 89 IU per 100 g (800 IU per quart) in plant-based yogurts or in soy beverage products.[129][130][131] Plant milks are defined as beverages made from soy, almond, rice, among other plant sources intended as alternatives to dairy milk.[132]
While some studies have found that vitamin D3 raises 25(OH)D blood levels faster and remains active in the body longer,[133][134] others contend that vitamin D2 sources are equally bioavailable and effective as D3 for raising and sustaining 25(OH)D.[122][135][136]
Food preparation
[edit]Vitamin D content in typical foods is reduced variably by cooking. Boiled, fried and baked foods retained 69–89% of original vitamin D.[137]
Recommended serum levels
[edit]
Recommendations on recommended 25(OH)D serum levels vary across authorities, and vary based on factors like age.[2] US labs generally report 25(OH)D levels in ng/mL.[140] Other countries often use nmol/L.[140] One ng/mL is approximately equal to 2.5 nmol/L.[141]
A 2014 review concluded that the most advantageous serum levels for 25(OH)D for all outcomes appeared to be close to 30 ng/mL (75 nmol/L).[142] The optimal vitamin D levels are still controversial and another review concluded that ranges from 30 to 40 ng/mL (75 to 100 nmol/L) were to be recommended for athletes.[143] Part of the controversy is because numerous studies have found differences in serum levels of 25(OH)D between ethnic groups; studies point to genetic as well as environmental reasons behind these variations.[144] Supplementation to achieve these standard levels could cause harmful vascular calcification.[35]
A 2012 meta-analysis showed that the risk of cardiovascular diseases increases when blood levels of vitamin D are lowest in a range of 8 to 24 ng/mL (20 to 60 nmol/L), although results among the studies analyzed were inconsistent.[145]
In 2011 an IOM committee concluded a serum 25(OH)D level of 20 ng/mL (50 nmol/L) is needed for bone and overall health. The dietary reference intakes for vitamin D are chosen with a margin of safety and 'overshoot' the targeted serum value to ensure the specified levels of intake achieve the desired serum 25(OH)D levels in almost all persons. No contributions to serum 25(OH)D level are assumed from sun exposure and the recommendations are fully applicable to people with dark skin or negligible exposure to sunlight. The Institute found serum 25(OH)D concentrations above 30 ng/mL (75 nmol/L) are "not consistently associated with increased benefit". Serum 25(OH)D levels above 50 ng/mL (125 nmol/L) may be cause for concern. However, some people with serum 25(OH)D between 30 and 50 ng/mL (75 nmol/L-125 nmol/L) will also have inadequate vitamin D.[41]
Excess
[edit]Vitamin D toxicity is rare.[30] It is caused by supplementing with high doses of vitamin D rather than sunlight. The threshold for vitamin D toxicity has not been established; however, according to some research:
- 100 μg/day (4k IU), have been shown to not cause toxic levels. ages 9–71[146]
- 240 μg/day (10k IU), over 5 months have been shown not to cause toxicity.[30]
- 1250 μg/day (50k IU) over several months can increase serum 25-hydroxyvitamin D levels to 150 ng/mL.[30][147]
Those with certain medical conditions, such as primary hyperparathyroidism,[148] are far more sensitive to vitamin D and develop hypercalcemia in response to any increase in vitamin D nutrition, while maternal hypercalcemia during pregnancy may increase fetal sensitivity to effects of vitamin D and lead to a syndrome of intellectual disability and facial deformities.[148][149]
Idiopathic infantile hypercalcemia is caused by a mutation of the CYP24A1 gene, leading to a reduction in the degradation of vitamin D. Infants who have such a mutation have an increased sensitivity to vitamin D and in case of additional intake a risk of hypercalcaemia.[150] The disorder can continue into adulthood.[151]
A review published in 2015 noted that adverse effects have been reported only at 25(OH)D serum concentrations above 200 nmol/L.[143]
Published cases of toxicity involving hypercalcemia in which the vitamin D dose and the 25-hydroxy-vitamin D levels are known all involve an intake of ≥40,000 IU (1,000 μg) per day.[148]
Those who are pregnant or breastfeeding should consult a doctor before taking a vitamin D supplement. The FDA advised manufacturers of liquid vitamin D supplements that droppers accompanying these products should be clearly and accurately marked for 400 international units (1 IU is the biological equivalent of 25 ng cholecalciferol/ergocalciferol). In addition, for products intended for infants, the FDA recommends the dropper hold no more than 400 IU.[152] For infants (birth to 12 months), the tolerable upper limit (maximum amount that can be tolerated without harm) is set at 25 μg/day (1,000 IU). One thousand micrograms per day in infants has produced toxicity within one month.[147] After being commissioned by the Canadian and American governments, the Institute of Medicine (IOM) as of 30 November 2010[update], has increased the tolerable upper limit (UL) to 2,500 IU per day for ages 1–3 years, 3,000 IU per day for ages 4–8 years and 4,000 IU per day for ages 9–71+ years (including pregnant or lactating women).[146]
Calcitriol itself is auto-regulated in a negative feedback cycle, and is also affected by parathyroid hormone, fibroblast growth factor 23, cytokines, calcium, and phosphate.[153]
A study published in 2017 assessed the prevalence of high daily intake levels of supplemental vitamin D among adults ages 20+ in the United States, based on publicly available NHANES data from 1999 through 2014. Its data shows the following:
- Over 18% of the population exceeds the NIH daily recommended allowance (RDA) of 600–800 IU,[2] by taking over 1000 IU, which suggests intentional supplement intake.[154]
- Over 3% of the population exceeds the NIH daily tolerable upper intake level (UL) of 4000 IU,[2] above which level the risk of toxic effects increases.[155][154]
- The percentage of the population taking over 1000 IU/day, as well as the percentage taking over 4000 IU/day, have both increased since 1999, according to trend analysis.[154]
Effect of excess
[edit]Vitamin D overdose causes hypercalcemia, which is a strong indication of vitamin D toxicity – this can be noted with an increase in urination and thirst. If hypercalcemia is not treated, it results in excess deposits of calcium in soft tissues and organs such as the kidneys, liver, and heart, resulting in pain and organ damage.[30][31][156]
The main symptoms of vitamin D overdose are hypercalcemia including anorexia, nausea, and vomiting. These may be followed by polyuria, polydipsia, weakness, insomnia, nervousness, pruritus and ultimately kidney failure. Furthermore, proteinuria, urinary casts, azotemia, and metastatic calcification (especially in the kidneys) may develop.[147] Other symptoms of vitamin D toxicity include intellectual disability in young children, abnormal bone growth and formation, diarrhea, irritability, weight loss, and severe depression.[30][156]
Vitamin D toxicity is treated by discontinuing vitamin D supplementation and restricting calcium intake. Kidney damage may be irreversible. Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity. The concentrations of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D produced is degraded.[148]
Biosynthesis
[edit]Synthesis of vitamin D in nature is dependent on the presence of UV radiation and subsequent activation in the liver and in the kidneys. Many animals synthesize vitamin D3 from 7-dehydrocholesterol, and many fungi synthesize vitamin D2 from ergosterol.[122][157]
Interactive pathway
[edit]Click on icon in lower right corner to open.
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
Photochemistry
[edit]

The transformation that converts 7-dehydrocholesterol to vitamin D3 occurs in two steps.[158][159] First, 7-dehydrocholesterol is photolyzed by ultraviolet light in a 6-electron conrotatory ring-opening electrocyclic reaction; the product is previtamin D3. Second, previtamin D3 spontaneously isomerizes to vitamin D3 (cholecalciferol) in an antarafacial sigmatropic [1,7] hydride shift. At room temperature, the transformation of previtamin D3 to vitamin D3 in an organic solvent takes about 12 days to complete. The conversion of previtamin D3 to vitamin D3 in the skin is about 10 times faster than in an organic solvent.[160]
The conversion from ergosterol to vitamin D2 follows a similar procedure, forming previtamin D2 by photolysis, which isomerizes to vitamin D2 (ergocalciferol).[161] The transformation of previtamin D2 to vitamin D2 in methanol has a rate comparable to that of previtamin D3. The process is faster in white button mushrooms.[122]: fig. 3
Synthesis in the skin
[edit]
Vitamin D3 is produced photochemically from 7-dehydrocholesterol in the skin of most vertebrate animals, including humans.[162] The precursor of vitamin D3, 7-dehydrocholesterol is produced in relatively large quantities. 7-Dehydrocholesterol reacts with UVB light at wavelengths of 290–315 nm.[163] These wavelengths are present in sunlight, as well as in the light emitted by the UV lamps in tanning beds (which produce ultraviolet primarily in the UVA spectrum, but typically produce 4% to 10% of the total UV emissions as UVB, some tanning beds can use only separate UVB light bulbs specifically for vitamin D production). Exposure to light through windows is insufficient because glass almost completely blocks UVB light.[164]
Adequate amounts of vitamin D can be produced with moderate sun exposure to the face, arms and legs (for those with the least melanin), averaging 5–30 minutes twice per week, or approximately 25% of the time for minimal sunburn. The darker the skin on the Fitzpatrick scale and the weaker the sunlight, the more minutes of exposure are needed. It also depends on parts of body exposed, all three factors affect minimal erythema dose (MED).[165] Vitamin D overdose from UV exposure is impossible: the skin reaches an equilibrium where the vitamin D degrades as fast as it is created.[30][166]
The skin consists of two primary layers: the inner layer called the dermis, and the outer, thinner epidermis. Vitamin D is produced in the keratinocytes of two innermost strata of the epidermis, the stratum basale and stratum spinosum, which also are able to produce calcitriol and express the VDR.[167]
Evolution
[edit]Vitamin D can be synthesized only by a photochemical process. Its production from sterols would have started very early in the evolution of life around the origin of photosynthesis, possibly helping to prevent DNA damage by absorbing UVB, making vitamin D an inactive end product. The familiar vitamin D endocrine machinery containing vitamin D receptor (VDR), various CYP450 enzymes for activation and inactivation, and a vitamin D binding protein (DBP) is found in vertebrates only. Primitive marine vertebrates are thought to absorb calcium from the ocean into their skeletons and eat plankton rich in vitamin D, although the function in those without a calcified cartilage is unclear.[168] Phytoplankton in the ocean (such as coccolithophore and Emiliania huxleyi) have been photosynthesizing vitamin D for more than 500 million years.
Land vertebrates required another source of vitamin D other than plants for their calcified skeletons. They had to either ingest it or be exposed to sunlight to photosynthesize it in their skin.[157][160] Land vertebrates have been photosynthesizing vitamin D for more than 350 million years.[169]
In birds and fur-bearing mammals, fur or feathers block UV rays from reaching the skin. Instead, vitamin D is created from oily secretions of the skin deposited onto the feathers or fur, and is obtained orally during grooming.[170] However, some animals, such as the naked mole-rat, are naturally cholecalciferol-deficient, as serum 25-OH vitamin D levels are undetectable.[171] Dogs and cats are practically incapable of vitamin D synthesis due to high activity of 7-dehydrocholesterol reductase, but get vitamin D from prey animals.[172]
Industrial synthesis
[edit]Vitamin D3 (cholecalciferol) is produced industrially by exposing 7-dehydrocholesterol to UVB and UVC light, followed by purification.[173][122] The 7-dehydrocholesterol is a natural substance in fish organs, especially the liver,[174] in wool grease (lanolin) from sheep and in some plants,[175] and lichen (Cladonia rangiferina).[176][177] Vitamin D2 (ergocalciferol) is produced in a similar way using ergosterol from yeast or mushrooms as a starting material.[173][122]
Mechanism of action
[edit]Metabolic activation
[edit]
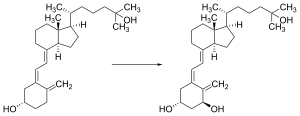
Vitamin D is carried via the blood to the liver, where it is converted into the prohormone calcifediol. Circulating calcifediol may then be converted into calcitriol – the biologically active form of vitamin D – in the kidneys.[178]
Whether synthesized in the skin or ingested, vitamin D is hydroxylated in the liver at position 25 (upper right of the molecule) to form 25-hydroxycholecalciferol (calcifediol or 25(OH)D).[3] This reaction is catalyzed by the microsomal enzyme vitamin D 25-hydroxylase, the product of the CYP2R1 human gene, and expressed by hepatocytes.[179] Once made, the product is released into the plasma, where it is bound to an α-globulin carrier protein named the vitamin D-binding protein.[180]
Calcifediol is transported to the proximal tubules of the kidneys, where it is hydroxylated at the 1-α position (lower right of the molecule) to form calcitriol (1,25-dihydroxycholecalciferol, 1,25(OH)2D).[1] The conversion of calcifediol to calcitriol is catalyzed by the enzyme 25-hydroxyvitamin D3 1-alpha-hydroxylase, which is the product of the CYP27B1 human gene.[1] The activity of CYP27B1 is increased by parathyroid hormone, and also by low calcium or phosphate.[1] Following the final converting step in the kidney, calcitriol is released into the circulation. By binding to vitamin D-binding protein, calcitriol is transported throughout the body, including to the intestine, kidneys, and bones.[18] Calcitriol is the most potent natural ligand of the vitamin D receptor, which mediates most of the physiological actions of vitamin D.[1][178] In addition to the kidneys, calcitriol is also synthesized by certain other cells, including monocyte-macrophages in the immune system. When synthesized by monocyte-macrophages, calcitriol acts locally as a cytokine, modulating body defenses against microbial invaders by stimulating the innate immune system.[178]
Inactivation
[edit]The activity of calcifediol and calcitriol can be reduced by hydroxylation at position 24 by vitamin D3 24-hydroxylase, forming secalciferol and calcitetrol, respectively.[3]
Difference between substrates
[edit]Vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol) share a similar mechanism of action as outlined above.[3] Metabolites produced by vitamin D2 are named with an er- or ergo- prefix to differentiate them from the D3-based counterparts (sometimes with a chole- prefix).[17]
- Metabolites produced from vitamin D2 tend to bind less well to the vitamin D-binding protein.[3]
- Vitamin D3 can alternatively be hydroxylated to calcifediol by sterol 27-hydroxylase (CYP27A1), but vitamin D2 cannot.[3]
- Ergocalciferol can be directly hydroxylated at position 24 by CYP27A1.[3] This hydroxylation also leads to a greater degree of inactivation: the activity of calcitriol decreases to 60% of original after 24-hydroxylation,[181] whereas ercalcitriol undergoes a 10-fold decrease in activity on conversion to ercalcitetrol.[182]
It is disputed whether these differences lead to a measurable drop in efficacy (see § Food fortification).
Intracellular mechanisms
[edit]Calcitriol enters the target cell and binds to the vitamin D receptor in the cytoplasm. This activated receptor enters the nucleus and binds to vitamin D response elements (VDRE) which are specific DNA sequences on genes.[1] Transcription of these genes is stimulated and produces greater levels of the proteins which mediate the effects of vitamin D.[3]
Some reactions of the cell to calcitriol appear to be too fast for the classical VDRE transcription pathway, leading to the discovery of various non-genomic actions of vitamin D. The membrane-bound PDIA3 likely serves as an alternate receptor in this pathway.[183] The classical VDR may still play a role.[184]
History
[edit]Vitamin D was discovered in 1922 following on from previous research.[185] American researchers Elmer McCollum and Marguerite Davis in 1914[11] discovered a substance in cod liver oil which later was called "vitamin A". British doctor Edward Mellanby noticed dogs that were fed cod liver oil did not develop rickets and concluded vitamin A, or a closely associated factor, could prevent the disease. In 1922, Elmer McCollum tested modified cod liver oil in which the vitamin A had been destroyed.[11] The modified oil cured the sick dogs, so McCollum concluded the factor in cod liver oil which cured rickets was distinct from vitamin A. He called it vitamin D because he thought it was the fourth vitamin to be named.[186][187] It was not initially realized that vitamin D can be synthesized by humans (in the skin) through exposure to UV light, and therefore is technically not a vitamin, but rather can be considered to be a hormone.
In 1925,[11] it was established that when 7-dehydrocholesterol is irradiated with light, a form of a fat-soluble substance is produced (now known as D3). Alfred Fabian Hess stated: "Light equals vitamin D."[188] Adolf Windaus, at the University of Göttingen in Germany, received the Nobel Prize in Chemistry in 1928 for his work on the constitution of sterols and their connection with vitamins.[189] In 1929, a group at NIMR in Hampstead, London, were working on the structure of vitamin D, which was still unknown, as well as the structure of steroids. A meeting took place with J.B.S. Haldane, J.D. Bernal, and Dorothy Crowfoot to discuss possible structures, which contributed to bringing a team together. X-ray crystallography demonstrated the sterol molecules were flat, not as proposed by the German team led by Windaus. In 1932, Otto Rosenheim and Harold King published a paper putting forward structures for sterols and bile acids which found immediate acceptance.[190] The informal academic collaboration between the team members Robert Benedict Bourdillon, Otto Rosenheim, Harold King, and Kenneth Callow was very productive and led to the isolation and characterization of vitamin D.[191] At this time, the policy of the Medical Research Council was not to patent discoveries, believing the results of medical research should be open to everybody. In the 1930s, Windaus clarified further the chemical structure of vitamin D.[192]
In 1923, American biochemist Harry Steenbock at the University of Wisconsin demonstrated that irradiation by ultraviolet light increased the vitamin D content of foods and other organic materials.[193] After irradiating rodent food, Steenbock discovered the rodents were cured of rickets. Using US$300 of his own money, Steenbock patented his invention. His irradiation technique was used for foodstuffs, most notably for milk. By the expiration of his patent in 1945, rickets had been all but eliminated in the US.[194]
In 1969, a specific binding protein for vitamin D called the vitamin D receptor was identified.[195] Shortly thereafter, the conversion of vitamin D to calcifediol and then to calcitriol, the biologically active form, was confirmed.[9][10][196] The photosynthesis of vitamin D3 in skin via previtamin D3 and its subsequent metabolism was described in 1980.[197]
Research
[edit]There is conflicting evidence about the benefits of interventions with vitamin D. Supplementation of between 800 and 1,000 IU is safe, but higher levels leading to blood levels of more than 50 ng/mL (125 nmol/L) may cause adverse effects.[2][198]
The US Office of Dietary Supplements established a Vitamin D Initiative over 2004–18 to track current research and provide education to consumers.[199] As of 2022, the role of vitamin D in the prevention and treatment of diabetes, glucose intolerance, hypertension, multiple sclerosis, and other medical conditions remains under preliminary research.[2]
Some preliminary studies link low vitamin D levels with disease later in life.[200] One meta-analysis found a decrease in mortality in elderly people.[12] Another meta-analysis covering over 350,000 people concluded that vitamin D supplementation in unselected community-dwelling individuals does not reduce skeletal (total fracture) or non-skeletal outcomes (myocardial infarction, ischemic heart disease, stroke, cerebrovascular disease, cancer) by more than 15%, and that further research trials with similar design are unlikely to change these conclusions.[14] As of 2022, there is insufficient evidence for an effect of vitamin D supplementation on the risk of cancer.[2][201][202] A 2019 meta-analysis found a small increase in risk of stroke when calcium and vitamin D supplements were taken together.[203]
COVID-19
[edit]As of September 2022[update] the US National Institutes of Health state there is insufficient evidence to recommend for or against using vitamin D supplementation to prevent or treat COVID-19.[204] The UK National Institute for Health and Care Excellence (NICE) does not recommend to offer a vitamin D supplement to people solely to prevent or treat COVID-19.[205][206] Both organizations included recommendations to continue the previous established recommendations on vitamin D supplementation for other reasons, such as bone and muscle health, as applicable. Both organizations noted that more people may require supplementation due to lower amounts of sun exposure during the pandemic.[204][205]
Several systematic reviews and meta-analyses of multiple studies have described the associations of vitamin D deficiency with adverse outcomes in COVID-19.[207][208][209][210][211][212] In the largest analysis, with data from 76 observational studies including almost two million adults, vitamin D deficiency or insufficiency significantly increased the susceptibility to becoming infected with COVID-19 and having severe COVID-19, with odds ratios of 1.5 and 1.9 respectively, but these findings had high risk of bias and heterogeneity. A two-fold greater mortality was found, but this analysis was less robust.[212] These findings confirm smaller, earlier analyses,[208][209][210][211] one of which, in reporting that people with COVID-19 tend to have lower 25(OH)D levels than healthy subjects, stated that the trend for associations with health outcomes was limited by the low quality of the studies and by the possibility of reverse causality mechanisms.[210]
A meta-analysis of three studies on the effect of oral vitamin D or calcifediol supplementation indicated a lower intensive care unit (ICU) admission rate (odds ratio: 0.36) compared to those without supplementation, but without a change in mortality.[213] A Cochrane review, also of three studies, found the evidence for the effectiveness of vitamin D supplementation for the treatment of COVID-19 to be very uncertain.[214] They found there was substantial clinical and methodological heterogeneity in the three studies that were included, mainly because of different supplementation strategies, vitamin D formulations (one using calcifediol), pre-treatment status and reported outcomes.[214] Another meta-analysis stated that the use of high doses of vitamin D in people with COVID-19 is not based on solid evidence although calcifediol supplementation may have a protective effect on ICU admissions.[210]
Other animals
[edit]Fish
[edit]Fish do not synthesise vitamin D in a natural setting and rely on dietary sources. As with mammals, vitamin D3 is more bioavailable than vitamin D2.[215] Unlike mammals, both hydroxylation steps from vitamin D3 to the active form 1,25 hydroxyvitamin D3 occur in the liver, so plasma levels of 25 hydroxyvitamin D3 is not an accurate measure of vitamin D3 levels.[215]
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v "Vitamin D". Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis. 11 February 2021. Archived from the original on 8 April 2015. Retrieved 14 March 2022.
- ^ a b c d e f g h i j k l m n o p q "Vitamin D: Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 12 August 2022. Archived from the original on 9 April 2021. Retrieved 22 February 2022.
- ^ a b c d e f g h i j k l Bikle DD (March 2014). "Vitamin D metabolism, mechanism of action, and clinical applications". Chemistry & Biology. 21 (3): 319–29. doi:10.1016/j.chembiol.2013.12.016. PMC 3968073. PMID 24529992.
- ^ Lehmann U, Gjessing HR, Hirche F, Mueller-Belecke A, Gudbrandsen OA, Ueland PM, et al. (October 2015). "Efficacy of fish intake on vitamin D status: a meta-analysis of randomized controlled trials". The American Journal of Clinical Nutrition. 102 (4): 837–47. doi:10.3945/ajcn.114.105395. PMID 26354531.
- ^ Cardwell, Glenn et al. “A Review of Mushrooms as a Potential Source of Dietary Vitamin D.” Nutrients vol. 10,10 1498. 13 Oct. 2018, doi:10.3390/nu10101498
- ^ Norman AW (August 2008). "From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health". The American Journal of Clinical Nutrition. 88 (2): 491S–9S. doi:10.1093/ajcn/88.2.491S. PMID 18689389.
- ^ "Vitamin D Tests". Lab Tests Online (USA). American Association for Clinical Chemistry. Archived from the original on 7 November 2017. Retrieved 23 June 2013.
- ^ Hollis BW (January 1996). "Assessment of vitamin D nutritional and hormonal status: what to measure and how to do it". Calcified Tissue International. 58 (1): 4–5. doi:10.1007/BF02509538. PMID 8825231. S2CID 35887181.
- ^ a b Holick MF, Schnoes HK, DeLuca HF (April 1971). "Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine". Proceedings of the National Academy of Sciences of the United States of America. 68 (4): 803–4. Bibcode:1971PNAS...68..803H. doi:10.1073/pnas.68.4.803. PMC 389047. PMID 4323790.
- ^ a b Norman AW, Myrtle JF, Midgett RJ, Nowicki HG, Williams V, Popják G (July 1971). "1,25-dihydroxycholecalciferol: identification of the proposed active form of vitamin D3 in the intestine". Science. 173 (3991): 51–4. Bibcode:1971Sci...173...51N. doi:10.1126/science.173.3991.51. PMID 4325863. S2CID 35236666.
- ^ a b c d Wolf G (June 2004). "The discovery of vitamin D: the contribution of Adolf Windaus". The Journal of Nutrition. 134 (6): 1299–302. doi:10.1093/jn/134.6.1299. PMID 15173387.
- ^ a b c d Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. (January 2014). "Vitamin D supplementation for prevention of mortality in adults". The Cochrane Database of Systematic Reviews (Systematic review). 1 (1): CD007470. doi:10.1002/14651858.CD007470.pub3. PMC 11285307. PMID 24414552.
- ^ a b Reid IR, Bolland MJ, Grey A (January 2014). "Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis". Lancet. 383 (9912): 146–55. doi:10.1016/s0140-6736(13)61647-5. PMID 24119980. S2CID 37968189.
- ^ a b c d e Bolland MJ, Grey A, Gamble GD, Reid IR (April 2014). "The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis". The Lancet. Diabetes & Endocrinology (Meta-analysis). 2 (4): 307–20. doi:10.1016/S2213-8587(13)70212-2. PMID 24703049.
- ^ "The Lancet Diabetes & Endocrinology: Vitamin D supplementation in adults does not prevent fractures, falls or improve bone mineral density". EurekAlert!. Archived from the original on 24 March 2022. Retrieved 23 February 2022.
The authors conclude that there is therefore little reason to use vitamin D supplements to maintain or improve musculoskeletal health, except for the prevention of rare conditions such as rickets and osteomalacia in high risk groups, which can be caused by vitamin D deficiency after long lack of exposure to sunshine.
- ^ Alayed Albarri EM, Sameer Alnuaimi A, Abdelghani D (4 August 2022). "Effectiveness of vitamin D2 compared with vitamin D3 replacement therapy in a primary healthcare setting: a retrospective cohort study". Qatar Medical Journal. 2022 (3): 29. doi:10.5339/qmj.2022.35. PMC 9372493. PMID 35974883.
Vitamin D is a fat-soluble vitamin consisting of vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol)
- ^ a b "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN): Nomenclature of vitamin D. Recommendations 1981". European Journal of Biochemistry. 124 (2): 223–7. May 1982. doi:10.1111/j.1432-1033.1982.tb06581.x. PMID 7094913.
- ^ a b c Fleet JC, Shapses SA (2020). "Vitamin D". In BP Marriott, DF Birt, VA Stallings, AA Yates (eds.). Present Knowledge in Nutrition, Eleventh Edition. London, United Kingdom: Academic Press (Elsevier). pp. 93–114. ISBN 978-0-323-66162-1.
- ^ Boron WF, Boulpaep EL (29 March 2016). Medical Physiology E-Book. Elsevier Health Sciences. ISBN 978-1-4557-3328-6. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ Bouillon R, Van Cromphaut S, Carmeliet G (February 2003). "Intestinal calcium absorption: Molecular vitamin D mediated mechanisms". Journal of Cellular Biochemistry. 88 (2): 332–9. doi:10.1002/jcb.10360. PMID 12520535. S2CID 9853381.
- ^ a b c Holick MF (December 2004). "Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1678S–88S. doi:10.1093/ajcn/80.6.1678S. PMID 15585788.
- ^ a b Bell TD, Demay MB, Burnett-Bowie SA (September 2010). "The biology and pathology of vitamin D control in bone". Journal of Cellular Biochemistry. 111 (1): 7–13. doi:10.1002/jcb.22661. PMC 4020510. PMID 20506379.
- ^ Watkins RR, Lemonovich TL, Salata RA (May 2015). "An update on the association of vitamin D deficiency with common infectious diseases". Canadian Journal of Physiology and Pharmacology. 93 (5): 363–8. doi:10.1139/cjpp-2014-0352. PMID 25741906.
- ^ Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK (February 1996). "Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells". Brain Research. Molecular Brain Research. 36 (1): 193–6. doi:10.1016/0169-328X(95)00314-I. PMID 9011759.
- ^ a b Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. (July 2011). "Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline". The Journal of Clinical Endocrinology and Metabolism. 96 (7): 1911–30. doi:10.1210/jc.2011-0385. PMID 21646368.
- ^ Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. (April 2016). "Vitamin D deficiency in Europe: pandemic?". The American Journal of Clinical Nutrition. 103 (4): 1033–44. doi:10.3945/ajcn.115.120873. PMC 5527850. PMID 26864360.
- ^ "Rickets". National Health Service. 8 March 2012. Archived from the original on 11 October 2017. Retrieved 9 July 2012.
- ^ Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. (February 2016). "Global Consensus Recommendations on Prevention and Management of Nutritional Rickets". The Journal of Clinical Endocrinology and Metabolism. 101 (2): 394–415. doi:10.1210/jc.2015-2175. PMC 4880117. PMID 26745253.
- ^ Eriksen EF, Glerup H (2002). "Vitamin D deficiency and aging: implications for general health and osteoporosis". Biogerontology. 3 (1–2): 73–7. doi:10.1023/A:1015263514765. PMID 12014847. S2CID 22112344.
- ^ a b c d e f g Holick MF (July 2007). "Vitamin D deficiency". The New England Journal of Medicine. 357 (3): 266–81. doi:10.1056/NEJMra070553. PMID 17634462. S2CID 18566028.
- ^ a b Brown JE, Isaacs J, Krinke B, Lechtenberg E, Murtaugh M (28 June 2013). Nutrition Through the Life Cycle. Cengage Learning. ISBN 978-1-285-82025-5. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ Schoenmakers I, Goldberg GR, Prentice A (June 2008). "Abundant sunshine and vitamin D deficiency". The British Journal of Nutrition. 99 (6): 1171–3. doi:10.1017/S0007114508898662. PMC 2758994. PMID 18234141.
- ^ Lowe NM, Bhojani I (June 2017). "Special considerations for vitamin D in the south Asian population in the UK". Therapeutic Advances in Musculoskeletal Disease. 9 (6): 137–44. doi:10.1177/1759720X17704430. PMC 5466148. PMID 28620422.
- ^ O'Connor MY, Thoreson CK, Ramsey NL, Ricks M, Sumner AE (2013). "The uncertain significance of low vitamin D levels in African descent populations: a review of the bone and cardiometabolic literature". Progress in Cardiovascular Diseases. 56 (3): 261–9. doi:10.1016/j.pcad.2013.10.015. PMC 3894250. PMID 24267433.
- ^ a b Freedman BI, Register TC (June 2012). "Effect of race and genetics on vitamin D metabolism, bone and vascular health". Nature Reviews. Nephrology. 8 (8): 459–66. doi:10.1038/nrneph.2012.112. PMC 10032380. PMID 22688752. S2CID 29026212.
- ^ Khalid AT, Moore CG, Hall C, Olabopo F, Rozario NL, Holick MF, et al. (September 2017). "Utility of sun-reactive skin typing and melanin index for discerning vitamin D deficiency". Pediatric Research. 82 (3): 444–51. doi:10.1038/pr.2017.114. PMC 5570640. PMID 28467404.
- ^ Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. (August 2009). "Vitamin D and calcium: a systematic review of health outcomes". Evidence Report/Technology Assessment (183): 1–420. PMC 4781105. PMID 20629479.
- ^ Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP (April 2014). "Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials". BMJ. 348: g2035. doi:10.1136/bmj.g2035. PMC 3972415. PMID 24690624.
- ^ a b Autier P, Boniol M, Pizot C, Mullie P (January 2014). "Vitamin D status and ill health: a systematic review". The Lancet. Diabetes & Endocrinology. 2 (1): 76–89. doi:10.1016/S2213-8587(13)70165-7. PMID 24622671.
- ^ Hussain S, Singh A, Akhtar M, Najmi AK (September 2017). "Vitamin D supplementation for the management of knee osteoarthritis: a systematic review of randomized controlled trials". Rheumatology International. 37 (9): 1489–98. doi:10.1007/s00296-017-3719-0. PMID 28421358. S2CID 23994681.
- ^ a b c d e f g h i j k l Institute of Medicine (2011). "8, Implications and Special Concerns". In Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds.). Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press. doi:10.17226/13050. ISBN 978-0-309-16394-1. PMID 21796828. S2CID 58721779. Archived from the original on 26 January 2021. Retrieved 17 September 2017.
- ^ a b Maxmen A (July 2011). "Nutrition advice: the vitamin D-lemma" (PDF). Nature. 475 (7354): 23–5. doi:10.1038/475023a. PMID 21734684. Archived (PDF) from the original on 3 August 2020. Retrieved 17 November 2011.
- ^ Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EH, Groot L, et al. (Consortium on Health Ageing: Network of Cohorts in Europe the United States) (June 2014). "Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States". BMJ. 348 (jun17 16): g3656. doi:10.1136/bmj.g3656. PMC 4061380. PMID 24938302.
- ^ Tuohimaa P (March 2009). "Vitamin D and aging". The Journal of Steroid Biochemistry and Molecular Biology. 114 (1–2): 78–84. doi:10.1016/j.jsbmb.2008.12.020. PMID 19444937. S2CID 40625040.
- ^ Tuohimaa P, Keisala T, Minasyan A, Cachat J, Kalueff A (December 2009). "Vitamin D, nervous system and aging". Psychoneuroendocrinology. 34 (Suppl 1): S278–86. doi:10.1016/j.psyneuen.2009.07.003. PMID 19660871. S2CID 17462970.
- ^ Elidrissy AT (September 2016). "The Return of Congenital Rickets, Are We Missing Occult Cases?". Calcified Tissue International (Review). 99 (3): 227–36. doi:10.1007/s00223-016-0146-2. PMID 27245342. S2CID 14727399.
- ^ Paterson CR, Ayoub D (October 2015). "Congenital rickets due to vitamin D deficiency in the mothers". Clinical Nutrition (Review). 34 (5): 793–8. doi:10.1016/j.clnu.2014.12.006. PMID 25552383.
- ^ Wagner CL, Greer FR (November 2008). "Prevention of rickets and vitamin D deficiency in infants, children, and adolescents". Pediatrics. 122 (5): 1142–52. doi:10.1542/peds.2008-1862. PMID 18977996. S2CID 342161.
- ^ Lerch C, Meissner T (October 2007). Lerch C (ed.). "Interventions for the prevention of nutritional rickets in term born children". The Cochrane Database of Systematic Reviews. 2010 (4): CD006164. doi:10.1002/14651858.CD006164.pub2. PMC 8990776. PMID 17943890.
- ^ Clements MR (1989). "The problem of rickets in UK Asians". Journal of Human Nutrition and Dietetics. 2 (2): 105–16. doi:10.1111/j.1365-277X.1989.tb00015.x.
- ^ Pettifor JM (December 2004). "Nutritional rickets: deficiency of vitamin D, calcium, or both?". The American Journal of Clinical Nutrition. 80 (6 Suppl): 1725S–9S. doi:10.1093/ajcn/80.6.1725S. PMID 15585795.
- ^ Dupuis EM (1 February 2002). Nature's Perfect Food: How Milk Became America's Drink. NYU Press. ISBN 978-0-8147-1938-1. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ Chibuzor MT, Graham-Kalio D, Osaji JO, Meremikwu MM, et al. (Cochrane Metabolic and Endocrine Disorders Group) (April 2020). "Vitamin D, calcium or a combination of vitamin D and calcium for the treatment of nutritional rickets in children". The Cochrane Database of Systematic Reviews. 2020 (4): CD012581. doi:10.1002/14651858.CD012581.pub2. PMC 7164979. PMID 32303107.
- ^ Holick MF (March 2006). "High prevalence of vitamin D inadequacy and implications for health". Mayo Clinic Proceedings. 81 (3): 353–73. doi:10.4065/81.3.353. PMID 16529140.
- ^ Avenell A, Mak JC, O'Connell D (April 2014). "Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men". The Cochrane Database of Systematic Reviews. 4 (4): CD000227. doi:10.1002/14651858.CD000227.pub4. PMC 7032685. PMID 24729336.
- ^ Bischoff-Ferrari HA, Willett WC, Orav EJ, Oray EJ, Lips P, Meunier PJ, et al. (July 2012). "A pooled analysis of vitamin D dose requirements for fracture prevention" (PDF). The New England Journal of Medicine. 367 (1): 40–9. doi:10.1056/NEJMoa1109617. hdl:1871/48765. PMID 22762317. S2CID 24338997. Archived (PDF) from the original on 15 December 2020. Retrieved 17 July 2019.
- ^ Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA (December 2011). "Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force". Annals of Internal Medicine. 155 (12): 827–38. doi:10.7326/0003-4819-155-12-201112200-00005. PMID 22184690. S2CID 22380502.
- ^ Zhao JG, Zeng XT, Wang J, Liu L (December 2017). "Association Between Calcium or Vitamin D Supplementation and Fracture Incidence in Community-Dwelling Older Adults: A Systematic Review and Meta-analysis". JAMA. 318 (24): 2466–2482. doi:10.1001/jama.2017.19344. PMC 5820727. PMID 29279934.
- ^ Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, et al. (August 2007). "Effectiveness and safety of vitamin D in relation to bone health". Evidence Report/Technology Assessment (158): 1–235. PMC 4781354. PMID 18088161.
- ^ Bolland MJ, Grey A, Gamble GD, Reid IR (July 2014). "Vitamin D supplementation and falls: a trial sequential meta-analysis". The Lancet. Diabetes & Endocrinology. 2 (7): 573–80. doi:10.1016/S2213-8587(14)70068-3. PMID 24768505.
- ^ Shuler FD, Wingate MK, Moore GH, Giangarra C (November 2012). "Sports health benefits of vitamin d". Sports Health. 4 (6): 496–501. doi:10.1177/1941738112461621. PMC 3497950. PMID 24179588.
- ^ a b Ben-Eltriki M, Gayle EJ, Paras JM, Nyame-Addo L, Chhabra M, Deb S (April 2024). "Vitamin D in Melanoma: Potential Role of Cytochrome P450 Enzymes". Life. 14 (4): 510. Bibcode:2024Life...14..510B. doi:10.3390/life14040510. PMC 11050855. PMID 38672780.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Zhao Y, Chen C, Pan W, Gao M, He W, Mao R, et al. (May 2016). "Comparative efficacy of vitamin D status in reducing the risk of bladder cancer: A systematic review and network meta-analysis". Nutrition. 32 (5): 515–523. doi:10.1016/j.nut.2015.10.023. PMID 26822497.
- ^ a b Hernández-Alonso P, Boughanem H, Canudas S, Becerra-Tomás N, Fernández de la Puente M, Babio N, et al. (July 2021). "Circulating vitamin D levels and colorectal cancer risk: A meta-analysis and systematic review of case-control and prospective cohort studies". Critical Reviews in Food Science and Nutrition. 63 (1): 1–17. doi:10.1080/10408398.2021.1939649. hdl:10609/136992. PMID 34224246. S2CID 235746547.
- ^ a b Sluyter JD, Manson JE, Scragg R (January 2021). "Vitamin D and Clinical Cancer Outcomes: A Review of Meta-Analyses". JBMR Plus. 5 (1): e10420. doi:10.1002/jbm4.10420. PMC 7839823. PMID 33553987.
- ^ Seraphin G, Rieger S, Hewison M, Capobianco E, Lisse TS (July 2023). "The impact of vitamin D on cancer: A mini review". J Steroid Biochem Mol Biol. 231: 106308. doi:10.1016/j.jsbmb.2023.106308. PMC 10330295. PMID 37054849.
- ^ Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E (May 2019). "Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials". Annals of Oncology. 30 (5): 733–743. doi:10.1093/annonc/mdz059. PMC 6821324. PMID 30796437.
- ^ Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, et al. (August 2019). "Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83 000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis". JAMA Cardiology. 4 (8): 765–76. doi:10.1001/jamacardio.2019.1870. PMC 6584896. PMID 31215980.
- ^ Nudy M, Krakowski G, Ghahramani M, Ruzieh M, Foy AJ (2020). "Vitamin D supplementation, cardiac events and stroke: A systematic review and meta-regression analysis". Int J Cardiol Heart Vasc. 28: 100537. doi:10.1016/j.ijcha.2020.100537. PMC 7240168. PMID 32462077.
- ^ Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. (May 2015). "Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data". JAMA Internal Medicine. 175 (5): 745–54. doi:10.1001/jamainternmed.2015.0237. PMC 5966296. PMID 25775274.
- ^ Zhang D, Cheng C, Wang Y, Sun H, Yu S, Xue Y, et al. (2020). "Effect of Vitamin D on Blood Pressure and Hypertension in the General Population: An Update Meta-Analysis of Cohort Studies and Randomized Controlled Trials". Prev Chronic Dis. 17: E03. doi:10.5888/pcd17.190307. PMC 6977781. PMID 31922371.
- ^ Abboud M, Al Anouti F, Papandreou D (2021). "Vitamin D status and blood pressure in children and adolescents: a systematic review of observational studies". Systematic Reviews. 10 (1): 60. doi:10.1186/s13643-021-01584-x. PMC 7898425. PMID 33618764.
- ^ Hewison M (2011). "Vitamin D and innate and adaptive immunity". Vitamins and the Immune System. Vitamins & Hormones. Vol. 86. Academic Press. pp. 23–62. doi:10.1016/B978-0-12-386960-9.00002-2. ISBN 978-0-12-386960-9. PMID 21419266.
- ^ Bishop E, Ismailova A, Dimeloe SK, Hewison M, White JH (August 2020). "Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory". JBMR Plus. 5 (1): e10405. doi:10.1002/jbm4.10405. PMC 7461279. PMID 32904944.
- ^ Nnoaham KE, Clarke A (February 2008). "Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis". International Journal of Epidemiology. 37 (1): 113–9. CiteSeerX 10.1.1.513.3969. doi:10.1093/ije/dym247. PMID 18245055.
- ^ Goyal JP, Singh S, Bishnoi R, Bhardwaj P, Kaur RJ, Dhingra S, et al. (July 2022). "Efficacy and safety of vitamin D in tuberculosis patients: a systematic review and meta-analysis". Expert Rev Anti Infect Ther. 20 (7): 1049–59. doi:10.1080/14787210.2022.2071702. PMID 35477334.
- ^ Cao Y, Wang X, Liu P, Su Y, Yu H, Du J (January 2022). "Vitamin D and the risk of latent tuberculosis infection: a systematic review and meta-analysis". BMC Pulm Med. 22 (1): 39. doi:10.1186/s12890-022-01830-5. PMC 8772077. PMID 35045861.
- ^ a b "SACN rapid review: Vitamin D and acute respiratory tract infections". Public Health England. Archived from the original on 14 January 2021. Retrieved 6 January 2021.
- ^ Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F (November 2015). "Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis". Inflammatory Bowel Diseases. 21 (11): 2708–17. doi:10.1097/MIB.0000000000000546. PMC 4615394. PMID 26348447.
- ^ a b c Wallace C, Gordon M, Sinopoulou V, Limketkai BN, et al. (Cochrane Gut Group) (October 2023). "Vitamin D for the treatment of inflammatory bowel disease". The Cochrane Database of Systematic Reviews. 2023 (10): CD011806. doi:10.1002/14651858.CD011806.pub2. PMC 10542962. PMID 37781953.
- ^ Guzman-Prado Y, Samson O, Segal JP, Limdi JK, Hayee B (May 2020). "Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis". Inflammatory Bowel Diseases. 26 (12): 1819–30. doi:10.1093/ibd/izaa087. PMID 32385487.
- ^ Jolliffe DA, Greenberg L, Hooper RL, Mathyssen C, Rafiq R, de Jongh RT, et al. (April 2019). "Vitamin D to prevent exacerbations of COPD: systematic review and meta-analysis of individual participant data from randomised controlled trials". Thorax. 74 (4): 337–45. doi:10.1136/thoraxjnl-2018-212092. PMID 30630893. S2CID 58548871.
- ^ Williamson A, Martineau AR, Sheikh A, Jolliffe D, Griffiths CJ (February 2023). "Vitamin D for the management of asthma". Cochrane Database Syst Rev. 2023 (2): CD011511. doi:10.1002/14651858.CD011511.pub3. PMC 9899558. PMID 36744416.
- ^ Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, et al. (July 2020). "Effects of Vitamin D Supplementation on Prevention of Type 2 Diabetes in Patients With Prediabetes: A Systematic Review and Meta-analysis". Diabetes Care. 43 (7): 1650–58. doi:10.2337/dc19-1708. PMID 33534730. S2CID 219897727.
- ^ Sahebi R, Rezayi M, Emadzadeh M, Salehi M, Tayefi M, Parizadeh SM, et al. (February 2019). "The effects of vitamin D supplementation on indices of glycemic control in Iranian diabetics: A systematic review and meta-analysis". Complementary Therapies in Clinical Practice. 34: 294–304. doi:10.1016/j.ctcp.2018.12.009. PMID 30712741. S2CID 57479957.
- ^ Mohammadi S, Hajhashemy Z, Saneei P (June 2021). "Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: a systematic review and dose-response meta-analysis of epidemiologic studies". Critical Reviews in Food Science and Nutrition. 2 (29): 8178–8198. doi:10.1080/10408398.2021.1926220. PMID 34076544. S2CID 235295924.
- ^ Brophy S, Davies H, Mannan S, Brunt H, Williams R (September 2011). "Interventions for latent autoimmune diabetes (LADA) in adults". The Cochrane Database of Systematic Reviews. 2011 (9): CD006165. doi:10.1002/14651858.cd006165.pub3. PMC 6486159. PMID 21901702.
- ^ Khoshbakht Y, Bidaki R, Salehi-Abargouei A (January 2018). "Vitamin D Status and Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Observational Studies". Advances in Nutrition. 9 (1): 9–20. doi:10.1093/advances/nmx002. PMC 6333940. PMID 29438455.
- ^ Gan J, Galer P, Ma D, Chen C, Xiong T (November 2019). "The Effect of Vitamin D Supplementation on Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Journal of Child and Adolescent Psychopharmacology. 29 (9): 670–87. doi:10.1089/cap.2019.0059. PMID 31368773. S2CID 199054851.
- ^ Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. (April 2014). "Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials". Psychosomatic Medicine. 76 (3): 190–6. doi:10.1097/psy.0000000000000044. PMC 4008710. PMID 24632894.
- ^ Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. (September 2012). "Vitamin D, cognition, and dementia: a systematic review and meta-analysis". Neurology. 79 (13): 1397–405. doi:10.1212/WNL.0b013e31826c197f. PMC 3448747. PMID 23008220.
- ^ a b Cui X, McGrath JJ, Burne TH, Eyles DW (July 2021). "Vitamin D and schizophrenia: 20 years on". Mol Psychiatry. 26 (7): 2708–20. doi:10.1038/s41380-021-01025-0. PMC 8505257. PMID 33500553.
- ^ Zhu JL, Luo WW, Cheng X, Li Y, Zhang QZ, Peng WX (June 2020). "Vitamin D deficiency and Schizophrenia in Adults: A Systematic Review and Meta-analysis of Observational Studies". Psychiatry Res. 288: 112959. doi:10.1016/j.psychres.2020.112959. PMID 32335466.
- ^ a b Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM (March 2013). "Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies". BMJ. 346: f1169. doi:10.1136/bmj.f1169. PMID 23533188.
- ^ a b Palacios C, De-Regil LM, Lombardo LK, Peña-Rosas JP (November 2016). "Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes". The Journal of Steroid Biochemistry and Molecular Biology. 164: 148–55. doi:10.1016/j.jsbmb.2016.02.008. PMC 5357731. PMID 26877200.
- ^ Roth DE, Leung M, Mesfin E, Qamar H, Watterworth J, Papp E (November 2017). "Vitamin D supplementation during pregnancy: state of the evidence from a systematic review of randomised trials". BMJ. 359: j5237. doi:10.1136/bmj.j5237. PMC 5706533. PMID 29187358.
- ^ a b Palacios C, Kostiuk LL, Cuthbert A, Weeks J (July 2024). "Vitamin D supplementation for women during pregnancy". The Cochrane Database of Systematic Reviews. 2024 (7): CD008873. doi:10.1002/14651858.CD008873.pub5. PMC 11287789. PMID 39077939.
- ^ Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW (March 2012). "Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus". Nutrients. 4 (3): 208–30. doi:10.3390/nu4030208. PMC 3347028. PMID 22666547.
- ^ Mallard SR, Howe AS, Houghton LA (October 2016). "Vitamin D status and weight loss: a systematic review and meta-analysis of randomized and nonrandomized controlled weight-loss trials". Am J Clin Nutr. 104 (4): 1151–59. doi:10.3945/ajcn.116.136879. PMID 27604772.
- ^ Alsharif SA, Baradwan S, Alshahrani MS, Khadawardi K, AlSghan R, Badghish E, et al. (2024). "Effect of Oral Consumption of Vitamin D on Uterine Fibroids: A Systematic Review and Meta-Analysis of Randomized Clinical Trials". Nutr Cancer. 76 (3): 226–35. doi:10.1080/01635581.2023.2288716. PMID 38234246.
{{cite journal}}: CS1 maint: overridden setting (link) - ^ Combs A, Singh B, Nylander E, Islam MS, Nguyen HV, Parra E, et al. (April 2023). "A Systematic Review of Vitamin D and Fibroids: Pathophysiology, Prevention, and Treatment". Reprod Sci. 30 (4): 1049–64. doi:10.1007/s43032-022-01011-z. PMID 35960442.
- ^ a b c European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) (2010). "Scientific opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular function (ID 159) pursuant to Article 13(1) of Regulation (EC) No 1924/2006". EFSA Journal. 8 (2): 1468–85. doi:10.2903/j.efsa.2010.1468.
- ^ European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA) (2011). "Scientific opinion on the substantiation of a health claim related to vitamin D and risk of falling pursuant to Article 14 of Regulation (EC) No 1924/2006" (PDF). EFSA Journal. 9 (9): 2382–2400. doi:10.2903/j.efsa.2011.2382. Archived (PDF) from the original on 20 August 2019. Retrieved 20 August 2019.
- ^ "Guidance for Industry: Food Labeling Guide". Food and Drug Administration (FDA). January 2013. Archived from the original on 22 December 2020. Retrieved 17 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Health Canada Scientific Summary on the U. S. Health Claim Regarding Calcium and Osteoporosis". Bureau of Nutritional Sciences Food Directorate, Health Products and Food Branch Health Canada. 1 May 2000. Archived from the original on 3 December 2016. Retrieved 29 January 2012.
- ^ Shimizu T (2002). "Newly established regulation in Japan: foods with health claims". Asia Pac J Clin Nutr. 11 (2): S94–6. doi:10.1046/j.1440-6047.2002.00007.x. PMID 12074195.
- ^ a b c d e Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes. Canberra: National Health and Medical Research Council. 2006. ISBN 1-86496-243-7. Archived from the original on 3 March 2023. Retrieved 19 March 2023.
- ^ a b c d "Vitamins and minerals – Vitamin D". National Health Service. 3 August 2020. Archived from the original on 30 October 2017. Retrieved 15 November 2020.
- ^ a b c d "Vitamin D and Calcium: Updated Dietary Reference Intakes". Nutrition and Healthy Eating. Health Canada. 5 December 2008. Archived from the original on 14 June 2017. Retrieved 28 April 2018.
- ^ a b c d EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (29 June 2016). "Dietary reference values for vitamin D". EFSA Journal. 14 (10): e04547. doi:10.2903/j.efsa.2016.4547. hdl:11380/1228918.
- ^ a b EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2012). "Scientific Opinion on the Tolerable Upper Intake Level of vitamin D". EFSA Journal (Submitted manuscript). 10 (7): 2813. doi:10.2903/j.efsa.2012.2813. hdl:2434/257871.
- ^ "PHE publishes new advice on vitamin D". Public Health England. 21 July 2016. Archived from the original on 3 January 2021. Retrieved 15 November 2020.
- ^ "Vitamin D". The Nutrition Source. 18 September 2012. Archived from the original on 13 April 2022. Retrieved 14 April 2022.
- ^ "Federal Register May 27, 2016 Food Labeling: Revision of the Nutrition and Supplement Facts Labels. FR page 33982" (PDF). Archived (PDF) from the original on 8 August 2016. Retrieved 20 August 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Daily Value Reference of the Dietary Supplement Label Database (DSLD)". Dietary Supplement Label Database (DSLD). Archived from the original on 7 April 2020. Retrieved 16 May 2020.
- ^ Salleh A (12 June 2012). "Vitamin D food fortification on the table". Australian Broadcasting Corporation. Archived from the original on 22 December 2020. Retrieved 12 June 2012.
- ^ "Australian Health Survey: Biomedical Results for Nutrients, 2011–12". Australian Bureau of Statistics. 21 December 2011. Archived from the original on 10 March 2023. Retrieved 19 March 2023.
- ^ "Vitamin D (translated)" (in Swedish). Swedish National Food Agency. Archived from the original on 26 October 2020. Retrieved 19 October 2018.
- ^ Vitamin-D-Bedarf bei fehlender endogener Synthese Deutsche Gesellschaft für Ernährung, January 2012
- ^ Pérez-López FR, Brincat M, Erel CT, Tremollieres F, Gambacciani M, Lambrinoudaki I, et al. (January 2012). "EMAS position statement: Vitamin D and postmenopausal health". Maturitas. 71 (1): 83–8. doi:10.1016/j.maturitas.2011.11.002. PMID 22100145.
- ^ Schmid A, Walther B (July 2013). "Natural vitamin D content in animal products". Advances in Nutrition. 4 (4): 453–62. doi:10.3945/an.113.003780. PMC 3941824. PMID 23858093.
- ^ a b c d e f g Keegan RJ, Lu Z, Bogusz JM, Williams JE, Holick MF (January 2013). "Photobiology of vitamin D in mushrooms and its bioavailability in humans". Dermato-Endocrinology. 5 (1): 165–76. doi:10.4161/derm.23321. PMC 3897585. PMID 24494050.
- ^ a b Wang T, Bengtsson G, Kärnefelt I, Björn LO (September 2001). "Provitamins and vitamins D2and D3in Cladina spp. over a latitudinal gradient: possible correlation with UV levels". Journal of Photochemistry and Photobiology B: Biology. 62 (1–2): 118–22. doi:10.1016/S1011-1344(01)00160-9. PMID 11693362. Archived from the original on 28 May 2020. Retrieved 31 October 2018.
- ^ Haytowitz DB (2009). "Vitamin D in mushrooms" (PDF). Nutrient Data Laboratory, US Department of Agriculture. Archived (PDF) from the original on 1 February 2021. Retrieved 16 April 2018.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Search, National Nutrient Database for Standard Reference Release 27". US Department of Agriculture, Agricultural Research Service. 2014. Archived from the original on 19 April 2014. Retrieved 12 June 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ de Lourdes Samaniego-Vaesken M, Alonso-Aperte E, Varela-Moreiras G (2012). "Vitamin food fortification today". Food & Nutrition Research. 56: 5459. doi:10.3402/fnr.v56i0.5459. PMC 3319130. PMID 22481896.
- ^ Spiro A, Buttriss JL (December 2014). "Vitamin D: An overview of vitamin D status and intake in Europe". Nutrition Bulletin. 39 (4): 322–350. doi:10.1111/nbu.12108. PMC 4288313. PMID 25635171.
- ^ "Vitamin D for Milk and Milk Alternatives". Food and Drug Administration (FDA). 15 July 2016. Archived from the original on 22 December 2020. Retrieved 22 February 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Federal Register: Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D2". Food and Drug Administration, US Department of Health and Human Services. 18 July 2016. Archived from the original on 22 December 2020. Retrieved 22 February 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "§172.379 Vitamin D2". Electronic Code of Federal Regulations. Archived from the original on 22 December 2020. Retrieved 16 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "§172.380 Vitamin D3". Electronic Code of Federal Regulations. Archived from the original on 22 December 2020. Retrieved 16 July 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Alternative to dairy milk". osoblanco. 16 January 2020. Archived from the original on 22 December 2020. Retrieved 20 January 2020.
- ^ Tripkovic L (2013). "Vitamin D2 vs. vitamin D3: Are they one and the same?". Nutrition Bulletin. 38 (2): 243–248. doi:10.1111/nbu.12029.
- ^ Alshahrani F, Aljohani N (September 2013). "Vitamin D: deficiency, sufficiency and toxicity". Nutrients. 5 (9): 3605–16. doi:10.3390/nu5093605. PMC 3798924. PMID 24067388.
- ^ Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF (March 2013). "Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation". The Journal of Clinical Endocrinology and Metabolism. 98 (3): 973–9. doi:10.1210/jc.2012-2114. PMC 3590486. PMID 23386645.
- ^ Borel P, Caillaud D, Cano NJ (2015). "Vitamin D bioavailability: state of the art" (PDF). Critical Reviews in Food Science and Nutrition. 55 (9): 1193–205. doi:10.1080/10408398.2012.688897. PMID 24915331. S2CID 9818323. Archived (PDF) from the original on 13 July 2021. Retrieved 27 April 2021.
- ^ Jakobsen J, Knuthsen P (April 2014). "Stability of vitamin D in foodstuffs during cooking". Food Chemistry. 148: 170–5. doi:10.1016/j.foodchem.2013.10.043. PMID 24262542.
- ^ Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. (29 August 2012). "A global representation of vitamin D status in healthy populations" (PDF). Archives of Osteoporosis. 7 (1–2): 155–172. doi:10.1007/s11657-012-0093-0. hdl:11343/220606. PMID 23225293. S2CID 207300035. Archived from the original on 19 March 2023. Retrieved 20 August 2019.
- ^ Wahl DA, Cooper C, Ebeling PR, Eggersdorfer M, Hilger J, Hoffmann K, et al. (1 February 2013). "A global representation of vitamin D status in healthy populations: reply to comment by Saadi". Archives of Osteoporosis. 8 (1–2): 122. doi:10.1007/s11657-013-0122-7. PMID 23371520. S2CID 5929230.
- ^ a b "25(OH)D levels in ng/mL". health harvard edu/. 19 December 2016. Archived from the original on 2 January 2020. Retrieved 2 January 2020.
- ^ "nmol converter". endmemo. Archived from the original on 2 February 2020. Retrieved 5 January 2020.
- ^ Bischoff-Ferrari HA (2008). "Optimal Serum 25-Hydroxyvitamin D Levels for Multiple Health Outcomes". Sunlight, Vitamin D and Skin Cancer (Review). Advances in Experimental Medicine and Biology. Vol. 810. Springer. pp. 500–25. doi:10.1007/978-0-387-77574-6_5. ISBN 978-0-387-77573-9. PMID 25207384.
- ^ a b Dahlquist DT, Dieter BP, Koehle MS (2015). "Plausible ergogenic effects of vitamin D on athletic performance and recovery". Journal of the International Society of Sports Nutrition (Review). 12: 33. doi:10.1186/s12970-015-0093-8. PMC 4539891. PMID 26288575.
- ^ Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, et al. (September 2008). "Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans". The Journal of Clinical Endocrinology and Metabolism. 93 (9): 3381–8. doi:10.1210/jc.2007-2702. PMC 2567851. PMID 18593774.
- ^ Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, et al. (November 2012). "Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies". Circulation: Cardiovascular Quality and Outcomes. 5 (6): 819–29. doi:10.1161/CIRCOUTCOMES.112.967604. PMC 3510675. PMID 23149428.
- ^ a b Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. (January 2011). "The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know". The Journal of Clinical Endocrinology and Metabolism. 96 (1): 53–8. doi:10.1210/jc.2010-2704. PMC 3046611. PMID 21118827.
- ^ a b c Vitamin D at The Merck Manual of Diagnosis and Therapy Professional Edition
- ^ a b c d Vieth R (May 1999). "Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety" (PDF). The American Journal of Clinical Nutrition. 69 (5): 842–56. doi:10.1093/ajcn/69.5.842. PMID 10232622. Archived (PDF) from the original on 3 July 2012. Retrieved 30 January 2011.
- ^ Tolerable Upper Intake Limits for Vitamins And Minerals (PDF). European Food Safety Authority. December 2006. ISBN 978-92-9199-014-6. Archived (PDF) from the original on 6 May 2019. Retrieved 27 February 2011.
- ^ De Paolis E, Scaglione GL, De Bonis M, Minucci A, Capoluongo E (October 2019). "CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: from genotype to phenotype". Clinical Chemistry and Laboratory Medicine. 57 (11): 1650–1667. doi:10.1515/cclm-2018-1208. PMID 31188746.
- ^ Tebben PJ, Singh RJ, Kumar R (October 2016). "Vitamin D-Mediated Hypercalcemia: Mechanisms, Diagnosis, and Treatment". Endocrine Reviews. 37 (5): 521–547. doi:10.1210/er.2016-1070. PMC 5045493. PMID 27588937.
- ^ "FDA Cautions on Accurate Vitamin D Supplementation for Infants" (Press release). Food and Drug Administration (FDA). 15 June 2010. Archived from the original on 12 January 2017.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Olmos-Ortiz A, Avila E, Durand-Carbajal M, Díaz L (January 2015). "Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes". Nutrients. 7 (1): 443–80. doi:10.3390/nu7010443. PMC 4303849. PMID 25584965.
- ^ a b c Rooney MR, Harnack L, Michos ED, Ogilvie RP, Sempos CT, Lutsey PL (June 2017). "Trends in Use of High-Dose Vitamin D Supplements Exceeding 1000 or 4000 International Units Daily, 1999–2014". JAMA. 317 (23): 2448–2450. doi:10.1001/jama.2017.4392. PMC 5587346. PMID 28632857.
- ^ Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium (2011). Ross AC, Taylor CL, Yaktine AL, Del Valle HB (eds.). Dietary Reference Intakes for Calcium and Vitamin D. The National Academies Collection: Reports funded by National Institutes of Health. Washington (DC): National Academies Press (US). PMID 21796828. Archived from the original on 29 January 2016. Retrieved 7 July 2012.
- ^ a b Insel P, Ross D, Bernstein M, McMahon K (18 March 2015). Discovering Nutrition. Jones & Bartlett Publishers. ISBN 978-1-284-06465-0. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ a b Holick MF (1992). "Evolutionary biology and pathology of vitamin D". J Nutr Sci Vitaminol (Tokyo). Spec No: 79–83. doi:10.3177/jnsv.38.special_79. PMID 1297827.
- ^ Holick MF (April 1987). "Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables". Federation Proceedings. 46 (5): 1876–82. PMID 3030826.
- ^ Deluca HF (January 2014). "History of the discovery of vitamin D and its active metabolites". BoneKEy Reports. 3: 479. doi:10.1038/bonekey.2013.213. PMC 3899558. PMID 24466410.
- ^ a b Holick MF (March 2004). "Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis". The American Journal of Clinical Nutrition. 79 (3): 362–71. doi:10.1093/ajcn/79.3.362. PMID 14985208.
- ^ Eyley SC, Williams DH (1975). "Photolytic production of vitamin D. The preparative value of a photo-sensitiser". Journal of the Chemical Society, Chemical Communications (20): 858a. doi:10.1039/C3975000858A.
- ^ Crissey SD, Ange KD, Jacobsen KL, Slifka KA, Bowen PE, Stacewicz-Sapuntzakis M, et al. (January 2003). "Serum concentrations of lipids, vitamin d metabolites, retinol, retinyl esters, tocopherols and selected carotenoids in twelve captive wild felid species at four zoos". The Journal of Nutrition. 133 (1): 160–6. doi:10.1093/jn/133.1.160. PMID 12514284.
- ^ Holick MF (2018). "Chapter 4: Photobiology of Vitamin D". In Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M (eds.). Vitamin D: Volume 1: Biochemistry, Physiology and Diagnostics (4th ed.). London, UK: Academic Press. ISBN 978-0-12-809965-0.
- ^ Holick MF (2020). "Sunlight, UV Radiation, Vitamin D, and Skin Cancer: How Much Sunlight do We Need?". Sunlight, Vitamin D and Skin Cancer. Advances in Experimental Medicine and Biology. Vol. 1268. Springer. pp. 19–36. doi:10.1007/978-3-030-46227-7_2. ISBN 978-3-030-46226-0. PMID 32918212. S2CID 221636019.
108 references
- ^ Young AR, Morgan KA, Harrison GI, Lawrence KP, Petersen B, Wulf HC, et al. (October 2021). "A revised action spectrum for vitamin D synthesis by suberythemal UV radiation exposure in humans in vivo". Proceedings of the National Academy of Sciences of the United States of America. 118 (40). Bibcode:2021PNAS..11815867Y. doi:10.1073/pnas.2015867118. PMC 8501902. PMID 34580202.
- ^ Holick MF (February 2002). "Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health". Current Opinion in Endocrinology, Diabetes and Obesity. 9 (1): 87–98. doi:10.1097/00060793-200202000-00011. S2CID 87725403.
- ^ Bikle DD (March 2010). "Vitamin D and the skin". Journal of Bone and Mineral Metabolism. 28 (2): 117–30. doi:10.1007/s00774-009-0153-8. PMID 20107849. S2CID 6072459.
- ^ Bouillon R, Suda T (January 2014). "Vitamin D: calcium and bone homeostasis during evolution". BoneKEy Reports. 3: 480. doi:10.1038/bonekey.2013.214. PMC 3899559. PMID 24466411.
- ^ Holick MF (1 April 2010). The Vitamin D Solution: A 3-Step Strategy to Cure Our Most Common Health Problems. Penguin Publishing Group. ISBN 978-1-101-22293-5. Archived from the original on 19 March 2023. Retrieved 19 March 2023.
- ^ Agarwal SC, Stout SD (28 June 2011). Bone Loss and Osteoporosis: An Anthropological Perspective. Springer Science & Business Media. ISBN 978-1-4419-8891-1. Archived (PDF) from the original on 29 January 2006.
The high 25(OH)D concentrations, and relatively high vitamin D requirements of apes and monkeys are understandable in light of their biology—their body surface area relative to mass is generally greater than for humans, and they are inveterate groomers, consuming by mouth the vitamin D generated from the oils secreted by skin into fur. Although much of the vitamin D produced within human skin is absorbed directly, birds and furbearing animals acquire most of their vitamin D orally, as they groom themselves (Bicknell and Prescott, 1946; Carpenter and Zhao, 1999). Vitamin D is generated from the oily secretions of skin into fur. The oral consumption of UV-exposed dermal excretion is the way many animals acquire the "nutrient," vitamin D. Although Fraser (1983) has argued that dermal absorption of vitamin D may be more natural, what we know from animals indicates that oral consumption is equally physiological. Since vitamin D can be extracted from UV-exposed human sweat and skin secretions (Bicknell and Prescott, 1946), it is also reasonable to think that early humans obtained some of their vitamin D by mouth as well, by licking the skin.
- ^ Yahav S, Buffenstein R (January 1993). "Cholecalciferol supplementation alters gut function and improves digestibility in an underground inhabitant, the naked mole rat (Heterocephalus glaber), when fed on a carrot diet". The British Journal of Nutrition. 69 (1): 233–41. doi:10.1079/BJN19930025. PMID 8384476.
- ^ Zafalon RV, Risolia LW, Pedrinelli V, Vendramini TH, Rodrigues RB, Amaral AR, et al. (January 2020). "Vitamin D metabolism in dogs and cats and its relation to diseases not associated with bone metabolism". Journal of Animal Physiology and Animal Nutrition. 104 (1): 322–42. doi:10.1111/jpn.13259. PMID 31803981.
- ^ a b Holick MF (November 2005). "The vitamin D epidemic and its health consequences" (PDF). The Journal of Nutrition. 135 (11): 2739S–2748S. doi:10.1093/jn/135.11.2739S. PMID 16251641. Archived (PDF) from the original on 18 November 2017. Retrieved 24 November 2011.
[Vitamin D3] is produced commercially by extracting 7-dehydrocholesterol from wool fat, followed by UVB irradiation and purification [...] [Vitamin D2] is commercially made by irradiating and then purifying the ergosterol extracted from yeast
- ^ Takeuchi A, Okano T, Sayamoto M, Sawamura S, Kobayashi T, Motosugi M, et al. (February 1986). "Tissue distribution of 7-dehydrocholesterol, vitamin D3 and 25-hydroxyvitamin D3 in several species of fishes". Journal of Nutritional Science and Vitaminology. 32 (1): 13–22. doi:10.3177/jnsv.32.13. PMID 3012050. Archived from the original on 1 November 2018. Retrieved 20 August 2019.
- ^ Jäpelt RB, Jakobsen J (May 2013). "Vitamin D in plants: a review of occurrence, analysis, and biosynthesis". Frontiers in Plant Science. 4: 136. doi:10.3389/fpls.2013.00136. PMC 3651966. PMID 23717318.
- ^ Göring H (November 2018). "Vitamin D in Nature: A Product of Synthesis and/or Degradation of Cell Membrane Components". Biochemistry. Biokhimiia. 83 (11): 1350–1357. doi:10.1134/S0006297918110056. PMID 30482146. S2CID 53437216.
- ^ Björn LO, Wang T (January 2000). "Vitamin D in an ecological context". International Journal of Circumpolar Health. 59 (1): 26–32. PMID 10850004.
- ^ a b c Adams JS, Hewison M (February 2010). "Update in vitamin D". The Journal of Clinical Endocrinology and Metabolism. 95 (2): 471–8. doi:10.1210/jc.2009-1773. PMC 2840860. PMID 20133466.
- ^ Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW (May 2004). "Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase". Proceedings of the National Academy of Sciences of the United States of America. 101 (20): 7711–5. Bibcode:2004PNAS..101.7711C. doi:10.1073/pnas.0402490101. PMC 419671. PMID 15128933.
- ^ Laing CJ, Cooke NE (2004). "Section I: Ch. 8: Vitamin D Binding Protein". In Feldman D, Glorieux FH, Pike JW (eds.). Vitamin D. Vol. 1 (2 ed.). Academic Press. pp. 117–134. ISBN 978-0-12-252687-9. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ Holick MF, Kleiner-Bossaller A, Schnoes HK, Kasten PM, Boyle IT, DeLuca HF (October 1973). "1,24,25-Trihydroxyvitamin D3. A metabolite of vitamin D3 effective on intestine". The Journal of Biological Chemistry. 248 (19): 6691–6. doi:10.1016/S0021-9258(19)43408-X. PMID 4355503.
- ^ Horst RL, Reinhardt TA, Ramberg CF, Koszewski NJ, Napoli JL (July 1986). "24-Hydroxylation of 1,25-dihydroxyergocalciferol. An unambiguous deactivation process". The Journal of Biological Chemistry. 261 (20): 9250–6. doi:10.1016/S0021-9258(18)67647-1. PMID 3013880.
- ^ Doroudi M, Schwartz Z, Boyan BD (March 2015). "Membrane-mediated actions of 1,25-dihydroxy vitamin D3: a review of the roles of phospholipase A2 activating protein and Ca(2+)/calmodulin-dependent protein kinase II". The Journal of Steroid Biochemistry and Molecular Biology. 147: 81–84. doi:10.1016/j.jsbmb.2014.11.002. PMC 4323845. PMID 25448737.
- ^ Hii CS, Ferrante A (March 2016). "The Non-Genomic Actions of Vitamin D". Nutrients. 8 (3): 135. doi:10.3390/nu8030135. PMC 4808864. PMID 26950144.
- ^ Jones G (April 2022). "100 YEARS OF VITAMIN D: Historical aspects of vitamin D". Endocrine Connections. 11 (4). doi:10.1530/EC-21-0594. PMC 9066576. PMID 35245207.
- ^ Carere S (25 July 2007). "Age-old children's disease back in force". Toronto Star. Archived from the original on 17 May 2008. Retrieved 24 August 2010.
- ^ McClean FC, Budy AM (28 January 1964). "Vitamin A, Vitamin D, Cartilage, Bones, and Teeth". Vitamins and Hormones. Vol. 21. Academic Press. pp. 51–52. ISBN 978-0-12-709821-0. Archived from the original on 19 March 2023. Retrieved 19 March 2023.
- ^ "History of Vitamin D". University of California at Riverside. 2011. Archived from the original on 16 October 2017. Retrieved 9 May 2014.
- ^ "Adolf Windaus – Biography". Nobelprize.org. 25 March 2010. Archived from the original on 24 July 2018. Retrieved 25 March 2010.
- ^ Rosenheim O, King H (1932). "The Ring-system of sterols and bile acids. Part II". J. Chem. Technol. Biotechnol. 51 (47): 954–7. doi:10.1002/jctb.5000514702.
- ^ Askew FA, Bourdillon RB, Bruce HM, Callow RK, St. L. Philpot J, Webster TA (1932). "Crystalline Vitamin D". Proceedings of the Royal Society of London. Series B, Containing Papers of a Biological Character. 109 (764): 488–506. doi:10.1098/rspb.1932.0008. JSTOR 81571.
- ^ Hirsch AL (2011). "Industrial aspects of vitamin D". In Feldman DJ, Pike JW, Adams JS (eds.). Vitamin D. Academic Press. p. 73. ISBN 978-0-12-387035-3. Archived from the original on 19 March 2023. Retrieved 19 March 2023.
- ^ Ziedonis AA, Mowery DC, Nelson RR, Bhaven NS (2004). Ivory tower and industrial innovation: university-industry technology transfer before and after the Bayh-Dole Act in the United States. Stanford Business Books. pp. 39–40. ISBN 978-0-8047-4920-6. Archived from the original on 19 March 2023. Retrieved 19 March 2023.
- ^ Marshall J (September 2010). Elbridge a Stuart: Founder of Carnation Company. Kessinger Publishing. ISBN 978-1-164-49678-6. Archived from the original on 19 March 2023. Retrieved 9 April 2017.
- ^ Haussler MR, Norman AW (January 1969). "Chromosomal receptor for a vitamin D metabolite". Proceedings of the National Academy of Sciences of the United States of America. 62 (1): 155–62. Bibcode:1969PNAS...62..155H. doi:10.1073/pnas.62.1.155. PMC 285968. PMID 5253652.
- ^ Holick MF, DeLuca HF, Avioli LV (January 1972). "Isolation and identification of 25-hydroxycholecalciferol from human plasma". Archives of Internal Medicine. 129 (1): 56–61. doi:10.1001/archinte.1972.00320010060005. PMID 4332591.
- ^ Holick MF, MacLaughlin JA, Clark MB, Holick SA, Potts JT, Anderson RR, et al. (October 1980). "Photosynthesis of previtamin D3 in human skin and the physiologic consequences". Science. 210 (4466): 203–5. Bibcode:1980Sci...210..203H. doi:10.1126/science.6251551. JSTOR 1685024. PMID 6251551.
- ^ Rizzoli R (January 2021). "Vitamin D supplementation: upper limit for safety revisited?". Aging Clin Exp Res (Review). 33 (1): 19–24. doi:10.1007/s40520-020-01678-x. PMC 7897606. PMID 32857334.
- ^ "ODS Vitamin D Initiative". Office of Dietary Supplements, US National Institutes of Health. 2018. Archived from the original on 26 January 2021. Retrieved 22 February 2023.
- ^ Pyrżak B, Witkowska-Sędek E, Krajewska M, Demkow U, Kucharska AM (2015). "Metabolic and immunological consequences of vitamin D deficiency in obese children". Body Metabolism and Exercise. Advances in Experimental Medicine and Biology. Vol. 840. Springer. pp. 13–9. doi:10.1007/5584_2014_81. ISBN 978-3-319-10249-8. PMID 25315624. S2CID 13296456.
- ^ "Vitamin D and cancer prevention". National Cancer Institute, US National Institutes of Health. 21 October 2013. Archived from the original on 13 February 2015. Retrieved 15 December 2016.
- ^ Goulão, Beatriz, Stewart, Fiona, Ford, John A., MacLennan, Graeme, Avenell, Alison (2018). "Cancer and vitamin D supplementation: a systematic review and meta-analysis". The American Journal of Clinical Nutrition. 107 (4): 652–63. doi:10.1093/ajcn/nqx047. PMID 29635490.
- ^ Khan SU, Khan MU, Riaz H, Valavoor S, Zhao D, Vaughan L, et al. (August 2019). "Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes: An Umbrella Review and Evidence Map". Annals of Internal Medicine. 171 (3): 190–98. doi:10.7326/m19-0341. PMC 7261374. PMID 31284304.
- ^ a b "Vitamin D". Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health (NIH). 26 September 2022. Retrieved 4 July 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b COVID-19 rapid guideline: vitamin D (PDF) (Technical report). National Institute for Health and Care Excellence (NICE). December 2020. ISBN 978-1-4731-3942-8. NG187. Archived from the original on 3 December 2021. Retrieved 22 February 2021.
- ^ Evidence reviews for the use of vitamin D supplementation as prevention and treatment of COVID-19 (PDF) (Report). National Institute for Health and Care Excellence (NICE). December 2020. Archived from the original on 20 October 2021. Retrieved 22 February 2021.
- ^ Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H (March 2021). "Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis". International Journal of Infectious Diseases. 104: 58–64. doi:10.1016/j.ijid.2020.12.077. PMC 7833186. PMID 33401034.
- ^ a b Kazemi A, Mohammadi V, Aghababaee SK, Golzarand M, Clark CC, Babajafari S (October 2021). "Association of Vitamin D Status with SARS-CoV-2 Infection or COVID-19 Severity: A Systematic Review and Meta-analysis". Advances in Nutrition. 12 (5): 1636–58. doi:10.1093/advances/nmab012. PMC 7989595. PMID 33751020.
- ^ a b Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A (July 2021). "Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies". The Journal of Steroid Biochemistry and Molecular Biology. 211: 105883. doi:10.1016/j.jsbmb.2021.105883. PMC 7997262. PMID 33775818.
- ^ a b c d Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G (June 2021). "The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis". Metabolism (Systematic review). 119: 154753. doi:10.1016/j.metabol.2021.154753. PMC 7989070. PMID 33774074.
- ^ a b Damascena AD, Azevedo LM, Oliveira TA, Santana JD, Pereira M (August 2021). "Addendum to vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis". Critical Reviews in Food Science and Nutrition. 63 (4): 557–62. doi:10.1080/10408398.2021.1951652. PMID 34384300. S2CID 236997712.
- ^ a b Dissanayake HA, de Silva NL, Sumanatilleke M, de Silva SD, Gamage KK, Dematapitiya C, et al. (April 2022). "Prognostic and Therapeutic Role of Vitamin D in COVID-19: Systematic Review and Meta-analysis". The Journal of Clinical Endocrinology and Metabolism. 107 (5): 1484–502. doi:10.1210/clinem/dgab892. PMC 8689831. PMID 34894254.
- ^ Shah K, Saxena D, Mavalankar D (January 2021). "Vitamin D supplementation, COVID-19 & Disease Severity: A meta-analysis". QJM: Monthly Journal of the Association of Physicians. 114 (3): 175–81. doi:10.1093/qjmed/hcab009. PMC 7928587. PMID 33486522.
- ^ a b Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, et al. (May 2021). "Vitamin D supplementation for the treatment of COVID-19: a living systematic review". The Cochrane Database of Systematic Reviews. 2021 (5): CD015043. doi:10.1002/14651858.CD015043. PMC 8406457. PMID 34029377. S2CID 235202971.
- ^ a b Cheng K, Huang Y, Wang C, Ali W, Karrow NA (September 2023). "Physiological function of vitamin D 3 in fish". Reviews in Aquaculture. 15 (4): 1732–1748. Bibcode:2023RvAq...15.1732C. doi:10.1111/raq.12814. ISSN 1753-5123.

