Cell culture: Difference between revisions
Tpbradbury (talk | contribs) rv vandalism from 23:38, 5 October 2011 |
|||
| Line 4: | Line 4: | ||
'''Cell culture''' is the complex process by which [[cell (biology)|cells]] are grown under controlled conditions. In practice, the term "cell culture" has come to refer to the culturing of cells derived from singlecellular [[eukaryote]]s, especially [[animal]] cells. However, there are also cultures of [[plant tissue culture|plants]], [[fungi]] and [[microbiological culture|microbes]], including [[viral culture|viruses]], [[bacteria]] and [[protist]]s. The historical development and methods of cell culture are closely interrelated to those of [[tissue culture]] and [[organ culture]]. |
'''Cell culture''' is the complex process by which [[cell (biology)|cells]] are grown under controlled conditions. In practice, the term "cell culture" has come to refer to the culturing of cells derived from singlecellular [[eukaryote]]s, especially [[animal]] cells. However, there are also cultures of [[plant tissue culture|plants]], [[fungi]] and [[microbiological culture|microbes]], including [[viral culture|viruses]], [[bacteria]] and [[protist]]s. The historical development and methods of cell culture are closely interrelated to those of [[tissue culture]] and [[organ culture]]. |
||
Animal cell culture became a common [[laboratory]] technique in the mid-1900s,<ref>{{cite web|url=http://www.bioteach.ubc.ca/Bioengineering/CellCulture/index.htm|title=Cell Culture|accessdate=2006-04-19}}</ref> but the concept of maintaining live cell lines separated from their original tissue source was discovered in the 19th century.<ref name="NIHtimeline">{{cite web|url=http://www.ncbi.nlm.nih.gov/books/bv.fcgi?db=Books&rid=mboc4.table.1516|title=Some landmarks in the development of tissue and cell culture.|accessdate=2006-04-19}}</ref> |
|||
Animal cell culture began when scientists removed a small amount of monkey kidney from a host organism and maintained it in a warm saline solution for several days, establishing the principle of tissue culture.<ref name="Zurlow">{{cite web|url=http://caat.jhsph.edu/pubsthe virus in monkey [[kidney]] cell cultures.}}</ref> |
|||
==History== |
|||
The 19th-century English physiologist [[Sydney Ringer]] developed [[Lactated Ringer's solution|salt solutions]] containing the chlorides of sodium, potassium, calcium and magnesium suitable for maintaining the beating of an isolated animal heart outside of the body.[http://www.whonamedit.com/synd.cfm/2119.html] In 1885, [[Wilhelm Roux]] removed a portion of the [[medullary plate]] of an [[embryo]]nic [[chicken]] and maintained it in a warm saline solution for several days, establishing the principle of tissue culture.<ref name="Zurlow">{{cite web|url=http://caat.jhsph.edu/pubs/animal_alts/appendix_c.htm|title=Animals and alternatives in testing.|accessdate=2006-04-19 |archiveurl = http://web.archive.org/web/20060225204205/http://caat.jhsph.edu/pubs/animal_alts/appendix_c.htm <!-- Bot retrieved archive --> |archivedate = 2006-02-25}}</ref> [[Ross Granville Harrison]], working at [[Johns Hopkins Medical School]] and then at [[Yale University]], published results of his experiments from 1907–1910, establishing the methodology of [[tissue culture]].<ref name="Schiff">Schiff, Judith Ann. {{cite web|title=An unsung hero of medical research.|url=http://www.yalealumnimagazine.com/issues/02_02/old_yale.html|accessdate=2006-04-19}} ''[[Yale Alumni Magazine]]'', February 2002.</ref> |
|||
Cell culture techniques were advanced significantly in the 1940s and 1950s to support research in [[virology]]. Growing viruses in cell cultures allowed preparation of purified viruses for the manufacture of [[vaccine]]s. The injectable [[polio vaccine#Inactivated vaccine|polio vaccine]] developed by [[Jonas Salk]] was one of the first products mass-produced using cell culture techniques. This vaccine was made possible by the cell culture research of [[John Franklin Enders]], [[Thomas Huckle Weller]], and [[Frederick Chapman Robbins]], who were awarded a [[Nobel Prize]] for their discovery of a method of growing the virus in monkey [[kidney]] cell cultures. |
|||
==Concepts in mammalian cell culture== |
==Concepts in mammalian cell culture== |
||
Revision as of 00:37, 27 January 2012

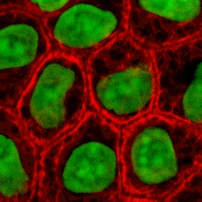
Cell culture is the complex process by which cells are grown under controlled conditions. In practice, the term "cell culture" has come to refer to the culturing of cells derived from singlecellular eukaryotes, especially animal cells. However, there are also cultures of plants, fungi and microbes, including viruses, bacteria and protists. The historical development and methods of cell culture are closely interrelated to those of tissue culture and organ culture.
Animal cell culture became a common laboratory technique in the mid-1900s,[1] but the concept of maintaining live cell lines separated from their original tissue source was discovered in the 19th century.[2]
History
The 19th-century English physiologist Sydney Ringer developed salt solutions containing the chlorides of sodium, potassium, calcium and magnesium suitable for maintaining the beating of an isolated animal heart outside of the body.[1] In 1885, Wilhelm Roux removed a portion of the medullary plate of an embryonic chicken and maintained it in a warm saline solution for several days, establishing the principle of tissue culture.[3] Ross Granville Harrison, working at Johns Hopkins Medical School and then at Yale University, published results of his experiments from 1907–1910, establishing the methodology of tissue culture.[4]
Cell culture techniques were advanced significantly in the 1940s and 1950s to support research in virology. Growing viruses in cell cultures allowed preparation of purified viruses for the manufacture of vaccines. The injectable polio vaccine developed by Jonas Salk was one of the first products mass-produced using cell culture techniques. This vaccine was made possible by the cell culture research of John Franklin Enders, Thomas Huckle Weller, and Frederick Chapman Robbins, who were awarded a Nobel Prize for their discovery of a method of growing the virus in monkey kidney cell cultures.
Concepts in mammalian cell culture
Isolation of cells
Cells can be isolated from tissues for ex vivo culture in several ways. Cells can be easily purified from blood; however, only the white cells are capable of growth in culture. Mononuclear cells can be released from soft tissues by enzymatic digestion with enzymes such as collagenase, trypsin, or pronase, which break down the extracellular matrix. Alternatively, pieces of tissue can be placed in growth media, and the cells that grow out are available for culture. This method is known as explant culture.
Cells that are cultured directly from a subject are known as primary cells. With the exception of some derived from tumors, most primary cell cultures have limited lifespan. After a certain number of population doublings (called the Hayflick limit), cells undergo the process of senescence and stop dividing, while generally retaining viability.
An established or immortalized cell line has acquired the ability to proliferate indefinitely either through random mutation or deliberate modification, such as artificial expression of the telomerase gene. Numerous cell lines are well established as representative of particular cell types.
Maintaining cells in culture
Cells are grown and maintained at an appropriate temperature and gas mixture (typically, 37°C, 5% CO2 for mammalian cells) in a cell incubator. Culture conditions vary widely for each cell type, and variation of conditions for a particular cell type can result in different phenotypes being expressed.
Aside from temperature and gas mixture, the most commonly varied factor in culture systems is the growth medium. Recipes for growth media can vary in pH, glucose concentration, growth factors, and the presence of other nutrients. The growth factors used to supplement media are often derived from animal blood, such as calf serum. One complication of these blood-derived ingredients is the potential for contamination of the culture with viruses or prions, particularly in medical biotechnology applications. Current practice is to minimize or eliminate the use of these ingredients wherever possible and use chemically defined media, but this cannot always be accomplished. Alternative strategies involve sourcing the animal blood from countries with minimum BSE/TSE risk, such as Australia and New Zealand, and using purified nutrient concentrates derived from serum in place of whole animal serum for cell culture.[5]
Plating density (number of cells per volume of culture medium) plays a critical role for some cell types. For example, a lower plating density makes granulosa cells exhibit estrogen production, while a higher plating density makes them appear as progesterone-producing theca lutein cells.[6]
Cells can be grown either in suspension or adherent cultures. Some cells naturally live in suspension, without being attached to a surface, such as cells that exist in the bloodstream. There are also cell lines that have been modified to be able to survive in suspension cultures so they can be grown to a higher density than adherent conditions would allow. Adherent cells require a surface, such as tissue culture plastic or microcarrier, which may be coated with extracellular matrix components to increase adhesion properties and provide other signals needed for growth and differentiation. Most cells derived from solid tissues are adherent. Another type of adherent culture is organotypic culture, which involves growing cells in a three-dimensional (3-D) environment as opposed to two-dimensional culture dishes. This 3D culture system is biochemically and physiologically more similar to in vivo tissue, but is technically challenging to maintain because of many factors (e.g. diffusion).
Cell line cross-contamination
Cell line cross-contamination can be a problem for scientists working with cultured cells. Studies suggest anywhere from 15–20% of the time, cells used in experiments have been misidentified or contaminated with another cell line.[7][8][9] Problems with cell line cross-contamination have even been detected in lines from the NCI-60 panel, which are used routinely for drug-screening studies.[10][11] Major cell line repositories, including the American Type Culture Collection (ATCC) and the German Collection of Microorganisms and Cell Cultures (DSMZ), have received cell line submissions from researchers that were misidentified by them.[10][12] Such contamination poses a problem for the quality of research produced using cell culture lines, and the major repositories are now authenticating all cell line submissions.[13] ATCC uses short tandem repeat (STR) DNA fingerprinting to authenticate its cell lines.[14]
To address this problem of cell line cross-contamination, researchers are encouraged to authenticate their cell lines at an early passage to establish the identity of the cell line. Authentication should be repeated before freezing cell line stocks, every two months during active culturing and before any publication of research data generated using the cell lines. Many methods are used to identify cell lines, including isoenzyme analysis, human lymphocyte antigen (HLA) typing, chromosomal analysis, karyotyping, morphology and STR analysis.[14]
One significant cell-line cross contaminant is the immortal HeLa cell line.
Manipulation of cultured cells
As cells generally continue to divide in culture, they generally grow to fill the available area or volume. This can generate several issues:
- Nutrient depletion in the growth media
- Accumulation of apoptotic/necrotic (dead) cells
- Cell-to-cell contact can stimulate cell cycle arrest, causing cells to stop dividing, known as contact inhibition.
- Cell-to-cell contact can stimulate cellular differentiation.
Among the common manipulations carried out on culture cells are media changes, passaging cells, and transfecting cells. These are generally performed using tissue culture methods that rely on sterile technique. Sterile technique aims to avoid contamination with bacteria, yeast, or other cell lines. Manipulations are typically carried out in a biosafety hood or laminar flow cabinet to exclude contaminating micro-organisms. Antibiotics (e.g. penicillin and streptomycin) and antifungals (e.g.amphotericin B) can also be added to the growth media.
As cells undergo metabolic processes, acid is produced and the pH decreases. Often, a pH indicator is added to the medium to measure nutrient depletion.
Media changes
In the case of adherent cultures, the media can be removed directly by aspiration, and then is replaced.
Passaging cells
Passaging (also known as subculture or splitting cells) involves transferring a small number of cells into a new vessel. Cells can be cultured for a longer time if they are split regularly, as it avoids the senescence associated with prolonged high cell density. Suspension cultures are easily passaged with a small amount of culture containing a few cells diluted in a larger volume of fresh media. For adherent cultures, cells first need to be detached; this is commonly done with a mixture of trypsin-EDTA; however, other enzyme mixes are now available for this purpose. A small number of detached cells can then be used to seed a new culture.
Transfection and transduction
Another common method for manipulating cells involves the introduction of foreign DNA by transfection. This is often performed to cause cells to express a protein of interest. More recently, the transfection of RNAi constructs have been realized as a convenient mechanism for suppressing the expression of a particular gene/protein. DNA can also be inserted into cells using viruses, in methods referred to as transduction, infection or transformation. Viruses, as parasitic agents, are well suited to introducing DNA into cells, as this is a part of their normal course of reproduction.
Established human cell lines
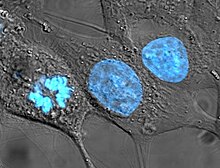
Cell lines that originate with humans have been somewhat controversial in bioethics, as they may outlive their parent organism and later be used in the discovery of lucrative medical treatments. In the pioneering decision in this area, the Supreme Court of California held in Moore v. Regents of the University of California that human patients have no property rights in cell lines derived from organs removed with their consent.[15]
Generation of hybridomas
It is possible to fuse normal cells with an immortalised cell line. This method is used to produce monoclonal antibodies. In brief, lymphocytes isolated from the spleen (or possibly blood) of an immunised animal are combined with an immortal myeloma cell line (B cell lineage) to produce a hybridoma which has the antibody specificity of the primary lymphoctye and the immortality of the myeloma. Selective growth medium (HA or HAT) is used to select against unfused myeloma cells; primary lymphoctyes die quickly in culture and only the fused cells survive. These are screened for production of the required antibody, generally in pools to start with and then after single cloning.
Applications of cell culture
Mass culture of animal cell lines is fundamental to the manufacture of viral vaccines and other products of biotechnology
Biological products produced by recombinant DNA (rDNA) technology in animal cell cultures include enzymes, synthetic hormones, immunobiologicals (monoclonal antibodies, interleukins, lymphokines), and anticancer agents. Although many simpler proteins can be produced using rDNA in bacterial cultures, more complex proteins that are glycosylated (carbohydrate-modified) currently must be made in animal cells. An important example of such a complex protein is the hormone erythropoietin. The cost of growing mammalian cell cultures is high, so research is underway to produce such complex proteins in insect cells or in higher plants, use of single embryonic cell and somatic embryos as a source for direct gene transfer via particle bombardment, transit gene expression and confocal microscopy observation is one of its applications. It also offers to confirm single cell origin of somatic embryos and the asymmetry of the first cell division, which starts the process.
Cell culture in two dimensions
Research in tissue engineering, stem cells and molecular biology primarily involves cultures of cells on flat plastic dishes. This technique is known as two-dimensional (2D) cell culture, and was first developed by Wilhelm Roux who, in 1885, removed a portion of the medullary plate of an embryonic chicken and maintained it in warm saline for several days on a flat glass plate. With the advance of polymer technology, today's standard plastic dish for 2D cell culture, commonly known as the Petri dish, was invented by Julius Richard Petri, a German bacteriologist, who is generally credited with inventing the Petri dish while working as assistant to Robert Koch.
Tissue culture and engineering
Cell culture is a fundamental component of tissue culture and tissue engineering, as it establishes the basics of growing and maintaining cells ex vivo. The major application of human cell culture is in stem cell industry, where mesenchymal stem cells can be cultured and cryopreserved for future use.
Vaccines
Vaccines for polio, measles, mumps, rubella, and chickenpox are currently made in cell cultures. Due to the H5N1 pandemic threat, research into using cell culture for influenza vaccines is being funded by the United States government. Novel ideas in the field include recombinant DNA-based vaccines, such as one made using human adenovirus (a common cold virus) as a vector,[16][17] and novel adjuvants.[18]
Culture of non-mammalian cells
Plant cell culture methods
Plant cell cultures are typically grown as cell suspension cultures in a liquid medium or as callus cultures on a solid medium. The culturing of undifferentiated plant cells and calli requires the proper balance of the plant growth hormones auxin and cytokinin.
Bacterial and yeast culture methods
For bacteria and yeasts, small quantities of cells are usually grown on a solid support that contains nutrients embedded in it, usually a gel such as agar, while large-scale cultures are grown with the cells suspended in a nutrient broth.
Viral culture methods
The culture of viruses requires the culture of cells of mammalian, plant, fungal or bacterial origin as hosts for the growth and replication of the virus. Whole wild type viruses, recombinant viruses or viral products may be generated in cell types other than their natural hosts under the right conditions. Depending on the species of the virus, infection and viral replication may result in host cell lysis and formation of a viral plaque.
Common cell lines
- Human cell lines
- HeLa
- National Cancer Institute's 60 cancer cell lines
- ESTDAB database
- DU145 (prostate cancer)
- Lncap (prostate cancer)
- MCF-7 (breast cancer)
- MDA-MB-438 (breast cancer)
- PC3 (prostate cancer)
- T47D (breast cancer)
- THP-1 (acute myeloid leukemia)
- U87 (glioblastoma)
- SHSY5Y Human neuroblastoma cells, cloned from a myeloma
- Saos-2 cells (bone cancer)
- Primate cell lines
- Vero (African green monkey Chlorocebus kidney epithelial cell line initiated in 1962)
- Rat tumor cell lines
- Mouse cell lines
- Plant cell lines
- Tobacco BY-2 cells (kept as cell suspension culture, they are model system of plant cell)
- Other species cell lines
- Zebrafish ZF4 and AB9 cells
- Madin-Darby canine kidney (MDCK) epithelial cell line
- Xenopus A6 kidney epithelial cells
List of cell lines
| Cell line | Meaning | Organism | Origin tissue | Morphology | Link | |
|---|---|---|---|---|---|---|
| 293-T | Human | Kidney (embryonic) | Derivative of HEK 293 ECACC Cell Lines Data Base (CLDB) | |||
| 3T3 cells | "3-day transfer, inoculum 3 x 10^5 cells" | Mouse | Embryonic fibroblast | Also known as NIH 3T3 ECACC. Search for the many 3T3 cells in the CLDB. | ||
| 721 | Human | Melanoma | ||||
| 9L | Rat | Glioblastoma | ||||
| A2780 | Human | Ovary | Ovarian cancer | ECACC, CLDB | ||
| A2780ADR | Human | Ovary | Adriamycin-resistant derivative | ECACC | ||
| A2780cis | Human | Ovary | Cisplatin-resistant derivative | ECACC | ||
| A172 | Human | Glioblastoma | Malignant glioma | ECACC | ||
| A20 | Murine | B lymphoma | B lymphocyte | |||
| A253 | Human | Head and neck carcinoma | Submandibular duct | |||
| A431 | Human | Skin epithelium | Squamous cell carcinoma | ECACCCLDB | ||
| A-549 | Human | Lungcarcinoma | Epithelium | DSMZECACC | ||
| ALC | Murine | Bone marrow | Stroma | PMID 2435412 | ||
| B16 | Murine | Melanoma | ECCAC | |||
| B35 | Rat | Neuroblastoma | ATCC | |||
| BCP-1 cells | Human | PBMC | HIV+ lymphoma | ATCC | ||
| BEAS-2B | Bronchial epithelium + Adenovirus 12-SV40 virus hybrid (Ad12SV40) | Human | Lung | Epithelial | ATCC | |
| bEnd.3 | Brain endothelial | Mouse | Brain/cerebral cortex | Endothelium | ATCC | |
| BHK-21 | Baby hamster kidney fibroblast cells | Hamster | Kidney | Fibroblast | ECACCOlympus | |
| BR 293 | Human | Breast | Breast cancer | |||
| BxPC3 | Biopsy xenograph of pancreatic carcinoma line 3 | Human | Pancreatic adenocarcinoma | Epithelial | ATCC | |
| C2C12 | Mouse | Myoblast cell line | ECACC | |||
| C3H-10T1/2 | Mouse | Embryonic mesenchymal cell line | ECACC | |||
| C6/36 | Asian tiger mosquito | Larval tissue | ECACC | |||
| Cal-27 | Human | Tongue | Squamous cell carcinoma | |||
| CHO | Chinese hamster ovary | Hamster | Ovary | Epithelium | ECACCICLC | |
| COR-L23 | Human | Lung | ECACC | |||
| COR-L23/CPR | Human | Lung | ECACC | |||
| COR-L23/5010 | Human | Lung | ECACC | |||
| COR-L23/R23 | Human | Lung | Epithelial | ECACC | ||
| COS-7 | Cercopithecus aethiops, origin-defective SV-40 | Ape - Cercopithecus aethiops (Chlorocebus) | Kidney | Fibroblast | ECACCATCC | |
| COV-434 | Human | Ovary | Metastatic granulosa cell carcinoma | [2]ECACC | ||
| CML T1 | Chronic myeloid leukaemia T lymphocyte 1 | Human | CML acute phase | T cell leukaemia | Blood | |
| CMT | Canine mammary tumor | Dog | Mammary gland | Epithelium | ||
| CT26 | Murine | Colorectal carcinoma | Colon | |||
| D17 | Canine | Osteosarcoma | ECACC | |||
| DH82 | Canine | Histiocytosis | Monocyte/macrophage | ECACC | ||
| DU145 | Human | Androgen insensitive carcinoma | Prostate | |||
| DuCaP | Dura mater cancer of the prostate | Human | Metastatic prostate cancer | Epithelial | PMID 11317521
{Ehrlich ascites carcinoma} mice | |
| EL4 | Mouse | T cell leukaemia | ECACC | |||
| EM2 | Human | CML blast crisis | Ph+ CML line | CLDB | ||
| EM3 | Human | CML blast crisis | Ph+ CML line | CLDB | ||
| EMT6/AR1 | Mouse | Breast | Epithelial-like | ECACC | ||
| EMT6/AR10.0 | Mouse | Breast | Epithelial-like | ECACC | ||
| FM3 | Human | Metastatic lymph node | Melanoma | |||
| H1299 | Human | Lung | Lung cancer | |||
| H69 | Human | Lung | ECACC | |||
| HB54 | Hybridoma | Hybridoma | Secretes L243 mAb (against HLA-DR) | Human Immunology | ||
| HB55 | Hybridoma | Hybridoma | secretes MA2.1 mAb (against HLA-A2 and HLA-B17) | Journal of Immunology | ||
| HCA2 | Human | Fibroblast | Journal of General Virology | |||
| HEK-293 | Human embryonic kidney | Human | Kidney (embryonic) | Epithelium | ATCC | |
| HeLa | "Henrietta Lacks" | Human | Cervical cancer | Epithelium | DSMZECACC | |
| Hepa1c1c7 | Clone 7 of clone 1 hepatoma line 1 | Mouse | Hepatoma | Epithelial | ECACC | |
| HL-60 | Human leukemia | Human | Myeloblast | Blood cells | ECACCDSMZ | |
| HMEC | Human mammary epithelial cell | Human | Epithelium | ECACC | ||
| HT-29 | Human | Colon epithelium | Adenocarcinoma | ECACC | ||
| Jurkat | Human | T cell leukemia | white blood cells | ECACC | ||
| J558L cells | Mouse | Myeloma | B lymphocyte cell | ECACC | ||
| JY cells | Human | Lymphoblastoid | EBV immortalised B cell | ECACC | ||
| K562 cells | Human | Lymphoblastoid | CML blast crisis | ECACC | ||
| Ku812 | Human | Lymphoblastoid | Erythroleukemia | ECACC | ||
| KCL22 | Human | Lymphoblastoid | CML | |||
| KG1 | Human | Lymphoblastoid | AML | |||
| KYO1 | Kyoto 1 | Human | Lymphoblastoid | CML | DSMZ | |
| LNCap | Lymph node cancer of the prostate | Human | Prostatic adenocarcinoma | Epithelial | ECACCATCC | |
| Ma-Mel 1, 2, 3....48 | Human | A range of melanoma cell lines | ||||
| MC-38 | Mouse | Adenocarcinoma | ||||
| MCF-7 | Michigan Cancer Foundation-7 | Human | Mammary gland | Invasive breast ductal carcinoma | ER+, PR+ | |
| MCF-10A | Michigan Cancer Foundation | Human | Mammary gland | Epithelium | ATCC | |
| MDA-MB-231 | M.D. Anderson - metastatic breast | Human | Breast | Cancer | ECACC | |
| MDA-MB-468 | M.D. Anderson - metastatic breast | Human | Breast | Cancer | ECACC | |
| MDA-MB-435 | M.D. Anderson - Metastatic Breast | Human | Breast | Melanoma or carcinoma (disputed) | Cambridge Pathology ECACC | |
| MDCK II | Madin Darby canine kidney | Dog | Kidney | Epithelium | ECACC ATCC | |
| MDCK II | Madin Darby canine kidney | Dog | Kidney | Epithelium | [3] ATCC | |
| MG63 | Human | Bone | Osteosarcoma | |||
| MOR/0.2R | Human | Lung | ECACC | |||
| MONO-MAC 6 | Human | WBC | Myeloid metaplasic AML | CLDB | ||
| MRC5 | Human (foetal) | Lung | Fibroblast] | |||
| MTD-1A | Mouse | Epithelium | ||||
| MyEnd | Myocardial endothelial | Mouse | Endothelium | |||
| NCI-H69/CPR | Human | Lung | ECACC | |||
| NCI-H69/LX10 | Human | Lung | ECACC | |||
| NCI-H69/LX20 | Human | Lung | ECACC | |||
| NCI-H69/LX4 | Human | Lung | ECACC | |||
| NIH-3T3 | NIH, 3-day transfer, inoculum 3 x 105 cells | Mouse | Embryo | Fibroblast | ECACCATCC | |
| NALM-1 | Peripheral blood | Blast-crisis CML | Cancer Genetics and Cytogenetics | |||
| NW-145 | Melanoma | ESTDAB | ||||
| OPCN / OPCT cell lines | Onyvax [4] prostate cancer.... | Range of prostate tumour lines | Asterand | |||
| Peer | Human | T cell leukemia | DSMZ | |||
| PNT-1A / PNT 2 | Prostate tumour lines | ECACC | ||||
| RBL cells | Rat Basophilic Leukaemia | Rat | Leukaemia | Basophil cell | ECACC | |
| RenCa | Renal carcinoma | Mouse | Renal carcinoma | |||
| RIN-5F | Mouse | Pancreas | ||||
| RMA/RMAS | Mouse | T cell tumour | ||||
| Saos-2 cells | Human | Osteosarcoma | ECACC | |||
| Sf-9 | Spodoptera frugiperda | Insect - Spodoptera frugiperda (moth) | Ovary | DSMZECACC | ||
| SiHa | Human | Cervical cancer | Epithelium | ECACC | ||
| SkBr3 | Human | Breast carcinoma | ||||
| T2 | Human | T cell leukemia/B cell line hybridoma | DSMZ | |||
| T-47D | Human | Mammary gland | Ductal carcinoma | |||
| T84 | Human | Colorectal carcinoma / Lung metastasis | Epithelium | ECACCATCC | ||
| THP1 cell line | Human | Monocyte | AML | ECACC | ||
| U373 | Human | Glioblastoma-astrocytoma | Epithelium | |||
| U87 | Human | Glioblastoma-astrocytoma | Epithelial-like | Abcam | ||
| U937 | Human | Leukaemic monocytic lymphoma | ECACC | |||
| VCaP | Vertebra prostate cancer | Human | Metastatic prostate cancer | Epithelial | ECACC ATCC | |
| Vero cells | Vero (truth) | African green monkey | Kidney epithelium | ECACC | ||
| WM39 | Human | Skin | Primary melanoma | |||
| WT-49 | Human | Lymphoblastoid | ||||
| X63 | Mouse | Melanoma | ||||
| YAC-1 | Mouse | Lymphoma | CLDB ECACC | |||
| YAR | Human | B cell | EBV transofrmed | [5] Human Immunology |
See also
- Biological immortality
- Cell culture assays
- Electric cell-substrate impedance sensing
- List of contaminated cell lines
- Organ culture
- Plant tissue culture
- Tissue culture
References and notes
- ^ "Cell Culture". Retrieved 2006-04-19.
- ^ "Some landmarks in the development of tissue and cell culture". Retrieved 2006-04-19.
- ^ "Animals and alternatives in testing". Archived from the original on 2006-02-25. Retrieved 2006-04-19.
- ^ Schiff, Judith Ann. "An unsung hero of medical research". Retrieved 2006-04-19. Yale Alumni Magazine, February 2002.
- ^ "LipiMAX purified lipoprotein solution from bovine serum". Selborne Biological Services. 2006. Retrieved 2010-02-02.
- ^ Portela VM, Zamberlam G, Price CA (2010). "Cell plating density alters the ratio of estrogenic to progestagenic enzyme gene expression in cultured granulosa cells". Fertil. Steril. 93 (6): 2050–5. doi:10.1016/j.fertnstert.2009.01.151. PMID 19324349.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Drexler, HG; Dirks, WG; Macleod, RA (1999). "False human hematopoietic cell lines: cross-contaminations and misinterpretations". Leukemia. 13 (10): 1601–7. doi:10.1038/sj/leu/2401510. ISSN 0887-6924. PMID 10516762.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Drexler, HG; Macleod, RA; Dirks, WG (2001). "Cross-contamination: HS-Sultan is not a myeloma but a Burkitt lymphoma cell line" (Free full text). Blood. 98 (12): 3495–6. doi:10.1182/blood.V98.12.3495. ISSN 0006-4971. PMID 11732505.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Cabrera, CM; Cobo, F; Nieto, A; Cortés, JL; Montes, RM; Catalina, P; Concha, A (2006). "Identity tests: determination of cell line cross-contamination". Cytotechnology. 51 (2): 45–50. doi:10.1007/s10616-006-9013-8. ISSN 0920-9069. PMID 19002894.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Chatterjee, R (2007). "Cell biology. Cases of mistaken identity". Science. 315 (5814): 928–31. doi:10.1126/science.315.5814.928. ISSN 0036-8075. PMID 17303729.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Liscovitch, M; Ravid, D (2007). "A case study in misidentification of cancer cell lines: MCF-7/AdrR cells (re-designated NCI/ADR-RES) are derived from OVCAR-8 human ovarian carcinoma cells". Cancer letters. 245 (1–2): 350–2. doi:10.1016/j.canlet.2006.01.013. ISSN 0304-3835. PMID 16504380.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Macleod, RA; Dirks, WG; Matsuo, Y; Kaufmann, M; Milch, H; Drexler, HG (1999). "Widespread intraspecies cross-contamination of human tumor cell lines arising at source". International journal of cancer. Journal international du cancer. 83 (4): 555–63. doi:10.1002/(SICI)1097-0215(19991112)83:4<555::AID-IJC19>3.0.CO;2-2. ISSN 0020-7136. PMID 10508494.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Masters, JR (2002). "HeLa cells 50 years on: the good, the bad and the ugly". Nature reviews. Cancer. 2 (4): 315–9. doi:10.1038/nrc775. ISSN 1474-175X. PMID 12001993.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Dunham, J.H.; Guthmiller, P. (2008). "Doing good science: Authenticating cell line identity" (PDF). Cell Notes. 22: 15–17.
- ^ Ceb.com
- ^ Reuters (2006-01-26). Wired.com "Quickie Bird Flu Vaccine Created". Wired. Retrieved 2010-01-31.
{{cite web}}:|author=has generic name (help); Check|url=value (help) - ^ Gao W, Soloff AC, Lu X, Montecalvo A, Nguyen DC, Matsuoka Y, Robbins PD, Swayne DE, Donis RO, Katz JM, Barratt-Boyes SM, Gambotto A. (2006). "Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization". Journal of Virology. 80 (4). United States: American Society for Microbiology: 1959–1964. doi:10.1128/JVI.80.4.1959-1964.2006. ISSN 0022-538X. PMC 1367171. PMID 16439551. Retrieved 2010-01-31.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "NIAID Taps Chiron to Develop Vaccine Against H9N2 Avian Influenza". National Institute of Allergy and Infectious Diseases (NIAID). 2004-08-17. Retrieved 2010-01-31. [dead link]
- Hpacultures.org.uk, Health Protection Agency Culture Collections (ECACC)
- MacLeod, R. A. F.; Dirks, Wilhelm G.; Matsuo, Yoshinobu; Kaufmann, Maren; Milch, Herbert; Drexler, Hans G. (1999). "Widespread intraspecies cross-contamination of human tumour cell lines". International Journal of Cancer. 83 (4): 555–563. doi:10.1002/(SICI)1097-0215(19991112)83:4<555::AID-IJC19>3.0.CO;2-2. PMID 10508494.
- Masters, John R. (2002). "HeLa cells 50 years on: the good, the bad and the ugly". Nature Reviews Cancer. 2 (4): 315–319. doi:10.1038/nrc775. PMID 12001993.
- Recently invented was the 3D Petri dish, the first 3D cell culture offering.
External links
- Table of common cell lines from Alberts 4th ed.
- Cancer Cells in Culture
- Hypertext version of the Cell Line Data Base
- Cell Culture Basics - Introduction to cell culture, covering topics such as laboratory set-up, safety and aseptic technique including basic cell culture protocols and video training
- Database of Who's Who in Cell Culture and Related Research
- Witkowski JA. Experimental pathology and the origins of tissue culture: Leo Loeb's contribution. Med Hist. 1983 July; 27(3): 269–288.
- Coriell Cell Repositories
- The National Centre for Cell Science (NCCS), Pune, India; national repository for cell lines/hybridomas etc.
- Neural Stem Cell Culture: Neurosphere generation, microscopical analysis and cryopreservation (a protocol)
- Rat Chromaffin cells primary cultures: Standardization and quality assessment for single-cell assays (a protocol)
