Menstrual cycle: Difference between revisions
Tags: Mobile edit Mobile web edit |
→Proliferative phase: Added cervical mucus is vaginal discharge Tags: Mobile edit Mobile web edit |
||
| Line 165: | Line 165: | ||
==== Proliferative phase ==== |
==== Proliferative phase ==== |
||
The proliferative phase is the second phase of the uterine cycle when estrogen causes the lining of the uterus to grow, or proliferate, during this time.<ref name="isbn0-07-303120-8">{{cite book | last1 = Losos | first1 = Jonathan B. | last2 = Raven | first2 = Peter H. | last3 = Johnson | first3 = George B. | last4 = Singer | first4 = Susan R. | title = Biology | publisher = McGraw-Hill | location = New York | year = 2002 | pages = 1207–09 | isbn = 978-0-07-303120-0 }}</ref> As they mature, the ovarian follicles secrete increasing amounts of [[estradiol]], and [[estrogen]]. The estrogens initiate the formation of a new layer of [[endometrium]] in the uterus, histologically identified as the proliferative endometrium. The estrogen also stimulates [[Crypt (anatomy)|crypts]] in the [[cervix]] to produce |
The proliferative phase is the second phase of the uterine cycle when estrogen causes the lining of the uterus to grow, or proliferate, during this time.<ref name="isbn0-07-303120-8">{{cite book | last1 = Losos | first1 = Jonathan B. | last2 = Raven | first2 = Peter H. | last3 = Johnson | first3 = George B. | last4 = Singer | first4 = Susan R. | title = Biology | publisher = McGraw-Hill | location = New York | year = 2002 | pages = 1207–09 | isbn = 978-0-07-303120-0 }}</ref> As they mature, the ovarian follicles secrete increasing amounts of [[estradiol]], and [[estrogen]]. The estrogens initiate the formation of a new layer of [[endometrium]] in the uterus, histologically identified as the proliferative endometrium. The estrogen also stimulates [[Crypt (anatomy)|crypts]] in the [[cervix]] to produce cervical mucus, which causes vaginal discharge regardless of arousal, and can be tracked by women practicing [[fertility awareness]].<ref name=tcoyf2>{{cite book | first=Toni | last=Weschler | year=2002 | title=Taking Charge of Your Fertility | pages=359–361 | edition=Revised | publisher=HarperCollins | location=New York | isbn=978-0-06-093764-5 }}</ref> |
||
==== Secretory phase ==== |
==== Secretory phase ==== |
||
Revision as of 23:12, 26 February 2019
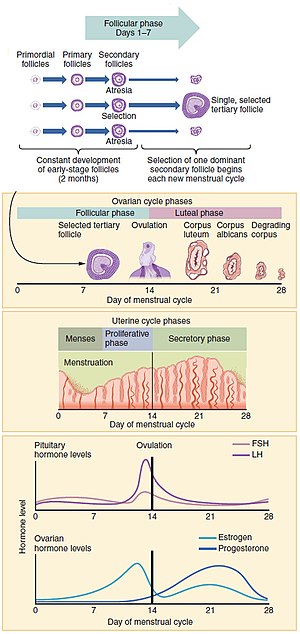
The menstrual cycle is the regular natural change that occurs in the female reproductive system (specifically the uterus and ovaries) that makes pregnancy possible.[1][2] The cycle is required for the production of oocytes, and for the preparation of the uterus for pregnancy.[1] Up to 80% of women report having some symptoms during the one to two weeks prior to menstruation.[3] Common symptoms include acne, tender breasts, bloating, feeling tired, irritability and mood changes.[4] These symptoms interfere with normal life and therefore qualify as premenstrual syndrome in 20 to 30% of women. In 3 to 8%, they are severe.[3]
The first period usually begins between twelve and fifteen years of age, a point in time known as menarche.[5] They may occasionally start as early as eight, and this onset may still be normal.[6] The average age of the first period is generally later in the developing world and earlier in developed world. The typical length of time between the first day of one period and the first day of the next is 21 to 45 days in young women and 21 to 35 days in adults (an average of 28 days[6][7][8]). Menstruation stops occurring after menopause which usually occurs between 45 and 55 years of age.[9] Bleeding usually lasts around 2 to 7 days.[6]
The menstrual cycle is governed by hormonal changes.[6] These changes can be altered by using hormonal birth control to prevent pregnancy.[10] Each cycle can be divided into three phases based on events in the ovary (ovarian cycle) or in the uterus (uterine cycle).[1] The ovarian cycle consists of the follicular phase, ovulation, and luteal phase whereas the uterine cycle is divided into menstruation, proliferative phase, and secretory phase.
Stimulated by gradually increasing amounts of estrogen in the follicular phase, discharges of blood (menses) flow stop, and the lining of the uterus thickens. Follicles in the ovary begin developing under the influence of a complex interplay of hormones, and after several days one or occasionally two become dominant (non-dominant follicles shrink and die). Approximately mid-cycle, 24–36 hours after the luteinizing hormone (LH) surges, the dominant follicle releases an ovocyte, in an event called ovulation. After ovulation, the ovocyte only lives for 24 hours or less without fertilization while the remains of the dominant follicle in the ovary become a corpus luteum; this body has a primary function of producing large amounts of progesterone. Under the influence of progesterone, the uterine lining changes to prepare for potential implantation of an embryo to establish a pregnancy. If implantation does not occur within approximately two weeks, the corpus luteum will involute, causing a sharp drop in levels of both progesterone and estrogen. The hormone drop causes the uterus to shed its lining in a process termed menstruation. Menstruation also occurs in closely related primates (apes and monkeys).[11]
Onset and frequency

The average age of menarche is 12–15.[5][12] They may occasionally start as early as eight, and this onset may still be normal.[6] This first period often occurs later in the developing world than the developed world.[8]
The average age of menarche is approximately 12.5 years in the United States,[13] 12.7 in Canada,[14] 12.9 in the UK[15] and 13.1 years in Iceland.[16] Factors such as genetics, diet and overall health can affect timing.[17]
The cessation of menstrual cycles at the end of a woman's reproductive period is termed menopause. The average age of menopause in women is 52 years, with anywhere between 45 and 55 being common. Menopause before age 45 is considered premature in industrialised countries.[18] Like the age of menarche, the age of menopause is largely a result of cultural and biological factors;[19] however, illnesses, certain surgeries, or medical treatments may cause menopause to occur earlier than it might have otherwise.[20]
The length of a woman's menstrual cycle typically varies somewhat, with some shorter cycles and some longer cycles. A woman who experiences variations of less than eight days between her longest cycles and shortest cycles is considered to have regular menstrual cycles. It is unusual for a woman to experience cycle length variations of more than four days. Length variation between eight and 20 days is considered as moderately irregular cycles. Variation of 21 days or more between a woman's shortest and longest cycle lengths is considered very irregular. [21]
The average menstrual cycle lasts 28 days. The variability of menstrual cycle lengths is highest for women under 25 years of age and is lowest, that is, most regular, for ages 25 to 39.[7] Subsequently, the variability increases slightly for women aged 40 to 44.[7]
The luteal phase of the menstrual cycle is about the same length in most individuals (mean 14.13 days, standard deviation 1.41 days)[22] whereas the follicular phase tends to show much more variability (log-normally distributed with 95% of individuals having follicular phases between 10.3 and 16.3 days).[23] The follicular phase also seems to get significantly shorter with age (geometric mean 14.2 days in women aged 18–24 vs. 10.4 days in women aged 40–44).[23]
Health effects
Some women with neurological conditions experience increased activity of their conditions at about the same time during each menstrual cycle. For example, drops in estrogen levels have been known to trigger migraines,[24] especially when the woman who suffers migraines is also taking the birth control pill. Many women with epilepsy have more seizures in a pattern linked to the menstrual cycle; this is called "catamenial epilepsy".[25] Different patterns seem to exist (such as seizures coinciding with the time of menstruation, or coinciding with the time of ovulation), and the frequency with which they occur has not been firmly established. Using one particular definition, one group of scientists found that around one-third of women with intractable partial epilepsy has catamenial epilepsy.[25][26][27] An effect of hormones has been proposed, in which progesterone declines and estrogen increases would trigger seizures.[28] Recently, studies have shown that high doses of estrogen can cause or worsen seizures, whereas high doses of progesterone can act like an antiepileptic drug.[29] Studies by medical journals have found that women experiencing menses are 1.68 times more likely to attempt suicide.[30]
Mice have been used as an experimental system to investigate possible mechanisms by which levels of sex steroid hormones might regulate nervous system function. During the part of the mouse estrous cycle when progesterone is highest, the level of nerve-cell GABA receptor subtype delta was high. Since these GABA receptors are inhibitory, nerve cells with more delta receptors are less likely to fire than cells with lower numbers of delta receptors. During the part of the mouse estrous cycle when estrogen levels are higher than progesterone levels, the number of delta receptors decrease, increasing nerve cell activity, in turn increasing anxiety and seizure susceptibility.[31]
Estrogen levels may affect thyroid behavior.[32] For example, during the luteal phase (when estrogen levels are lower), the velocity of blood flow in the thyroid is lower than during the follicular phase (when estrogen levels are higher).[33]
Among women living closely together, it was once thought that the onsets of menstruation tend to synchronize. This effect was first described in 1971, and possibly explained by the action of pheromones in 1998.[34] Subsequent research has called this hypothesis into question.[35]
Research indicates that women have a significantly higher likelihood of anterior cruciate ligament injuries in the pre-ovulatory stage, than post-ovulatory stage.[36]
Fertility
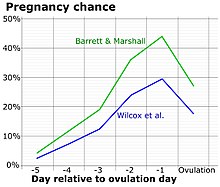
The most fertile period (the time with the highest likelihood of pregnancy resulting from sexual intercourse) covers the time from some 5 days before until 1 to 2 days after ovulation.[38] In a 28‑day cycle with a 14‑day luteal phase, this corresponds to the second and the beginning of the third week. A variety of methods have been developed to help individual women estimate the relatively fertile and the relatively infertile days in the cycle; these systems are called fertility awareness.
There are many fertility testing methods, including urine test kits that detect the LH surge that occurs 24 to 36 hours before ovulation; these are known as ovulation predictor kits (OPKs).[39] Computerized devices that interpret basal body temperatures, urinary test results, or changes in saliva are called fertility monitors. Fertility awareness methods that rely on cycle length records alone are called calendar-based methods.[40] Methods that require observation of one or more of the three primary fertility signs (basal body temperature, cervical mucus, and cervical position)[41] are known as symptoms-based methods.[40]
A woman's fertility is also affected by her age.[42] As a woman's total egg supply is formed in fetal life,[43] to be ovulated decades later, it has been suggested that this long lifetime may make the chromatin of eggs more vulnerable to division problems, breakage, and mutation than the chromatin of sperm, which are produced continuously during a man's reproductive life. However, despite this hypothesis, a similar paternal age effect has also been observed.
As measured on women undergoing in vitro fertilization, a longer menstrual cycle length is associated with higher pregnancy and delivery rates, even after age adjustment.[44] Delivery rates after IVF have been estimated to be almost doubled for women with a menstrual cycle length of more than 34 days compared with women with a menstrual cycle length shorter than 26 days.[44] A longer menstrual cycle length is also significantly associated with better ovarian response to gonadotropin stimulation and embryo quality.[44]
Mood and behavior
The different phases of the menstrual cycle correlate with women's moods. In some cases, hormones released during the menstrual cycle can cause behavioral changes in females; mild to severe mood changes can occur.[45] The menstrual cycle phase and ovarian hormones may contribute to increased empathy in women. The natural shift of hormone levels during the different phases of the menstrual cycle has been studied in conjunction with test scores. When completing empathy exercises, women in the follicular stage of their menstrual cycle performed better than women in their midluteal phase. A significant correlation between progesterone levels and the ability to accurately recognize emotion was found. Performances on emotion recognition tasks were better when women had lower progesterone levels. Women in the follicular stage showed higher emotion recognition accuracy than their midluteal phase counterparts. Women were found to react more to negative stimuli when in midluteal stage over the women in the follicular stage, perhaps indicating more reactivity to social stress during that menstrual cycle phase.[46] Overall, it has been found that women in the follicular phase demonstrated better performance in tasks that contain empathetic traits.
Fear response in women during two different points in the menstrual cycle has been examined. When estrogen is highest in the preovulatory stage, women are significantly better at identifying expressions of fear than women who were menstruating, which is when estrogen levels are lowest. The women were equally able to identify happy faces, demonstrating that the fear response was a more powerful response. To summarize, menstrual cycle phase and the estrogen levels correlates with women’s fear processing.[47]
However, the examination of daily moods in women with measuring ovarian hormones may indicate a less powerful connection. In comparison to levels of stress or physical health, the ovarian hormones had less of an impact on overall mood.[48] This indicates that while changes of ovarian hormones may influence mood, on a day-to-day level it does not influence mood more than other stressors do.
Sexual feelings and behaviors change during the menstrual cycle. Before and during ovulation, high levels of estrogen and androgens result in women having a relatively increased interest in sexual activity.[49] Unlike other mammals, women may show some interest in preferred sexual activity across all days of the menstrual cycle, regardless of fertility.[50]
A recent study shows that language seems be affected by the menstrual cycle [51]
Mate choice
Behavior towards potential mating partners changes during different phases of the menstrual cycle.[52][53][54] Near ovulation, women may have increased physical attraction and interest in attending social gatherings with men.[55] During the fertile phase of the cycle, women appear to prefer males who are more masculine.[56] The intensity of mate guarding differs across the phases of the cycle, with increased mate guarding occurring when women are fertile.[54][57][58]
During the fertile phase, many women experience more attraction, fantasies and sexual interest for extra pair men but not for the primary partner.[55][54][59] They also engage in extra-pair flirtations and demonstrate a preference for extra pair copulation.[55][59]
Voice
Preferences for voice pitch change across the cycle.[59] When seeking a short term mating partner, women may prefer a male with a low voice pitch, particularly during the fertile phase.[59] During the late follicular phase, it is common for women demonstrate a preference for mates with a masculine, deep voice.[60] Research has also been conducted on the attractiveness of the female voice throughout the cycle.[61] During their most fertile phase of the menstrual cycle, there is some evidence that female voices are rated as significantly more attractive.[61] This effect is not found with women on the birth control pill.[61]
Smell
Women's preference for male's body odor can change across the menstrual cycle.[62] Males who score highly on dominance have been rated as sexier by females during the fertile phase of the menstrual cycle. Additionally, during their most fertile phase of the menstrual cycle, women may show preference for the odor of symmetrical men.[54] This effect is not found for women on the birth control pill.[63] Also, during the late follicular and ovulatory phases, women prefer the scent of masculine men.[59] The scent of androsterone (responsible for testosterone levels) is highly preferred by women during the peak of their fertility in the menstrual cycle.[59] Moreover, women may demonstrate preference for men with a scent that indicates developmental stability.[59]
With regard to women's smell across the cycle, some evidence indicates that men use olfactory cues in order to know if a woman is ovulating.[62] Using a rating of women's odors, women who are ovulating have been rated as more attractive by men.[62] Men demonstrate preferences for the scent of fertile women.[62]
Body
Preferences for facial features in mates can also change across the cycle.[59] There has been no difference found in preference for long-term mating partners during the menstrual cycle; however, those seeking a short-term relationship were more likely to choose a partner with more masculine features than usual.[55][60] This was found to be the case especially during the woman's high conception risk stage and when salivary testosterone was high.[64] However, when women are in the luteal (non-fertile) phase, they tend to prefer men (and females) with more feminine faces.[60] A preference is also shown for self-resembling faces and apparent health in faces during the luteal phase of the cycle.[65] Apparent health preferences were found to be strongest when progesterone levels were high.[65] Additionally, during the fertile phase, many women show a preference for men with darker skin pigmentation.[59] Research on facial symmetry is mixed.[66]
Preferences for body features can change during the fertile phase of the cycle. Women seeking a short-term partner demonstrate a preference for taller and muscular males.[59] Women also show preferences of males with masculine bodies at peak fertility.[59][64] Mixed research has been found regarding body symmetry preferences throughout different phases of the cycle.[59]
Personality
In short term mates, during the fertile phase, women may show more attraction to dominant men who display social presence.[59] For long-term mates, shifts in desired trait preferences do not occur throughout the cycle.[59]
Eating behavior
Females have been found to experience different eating habits at different stages of their menstrual cycle, with food intake being higher during the luteal phase than the follicular phase.[67][68] Food intake increases by approximately 10% during the luteal phase compared to the follicular phase.[68]
Various studies have shown that during the luteal phase woman consume more carbohydrates, proteins and fats and that 24-hour energy expenditure shows increases between 2.5-11.5%.[69] The increasing intake during the luteal phase may be related to higher preferences for sweet and fatty foods, which occurs naturally and is enhanced during the luteal phases of the menstrual cycle.[69] This is due to the higher metabolic demand during this phase.[70] In particular, women tend to show a cravings for chocolate, with higher cravings during the luteal phase.[69]
Females with premenstrual syndrome (PMS) report changes in appetite across the menstrual cycle more than non-sufferers of PMS, possibly due to their oversensitivity to changes in hormone levels.[68] In women with PMS, food intake is higher in the luteal phase than follicular.[71] The remaining symptoms of PMS, including mood changes and physical symptoms, also occur during the luteal phase. No difference for preference of food types has been found between PMS sufferers and non-sufferers.[67]
The different levels of ovarian hormones at different stages of the cycle have been used to explain eating behaviour changes. Progesterone has been shown to promote fat storage, causing a higher intake of fatty foods during the luteal phase when progesterone levels are higher.[68] Additionally, with a high estrogen level dopamine is ineffective in converting to noradrenaline, a hormone which promotes eating, therefore decreasing appetite.[68] In humans, the level of these ovarian hormones during the menstrual cycle have been found to influence binge eating.[72]
It is theorized that the use of birth control pills should affect eating behaviour as they minimise or remove the fluctuations in hormone levels.[67] The neurotransmitter serotonin is also thought to play a role in food intake. Serotonin is responsible for inhibiting eating and controlling meal size,[73] among other things, and is modulated in part by ovarian hormones.[74]
A number of factors affect whether dieting will affect these menstrual processes: age, weight loss and the diet itself. First, younger women are likely to experience menstrual irregularities due to their diet. Second, menstrual abnormalities are more likely with more weight loss. For example, anovulatory cycles can occur as a result of adopting a restricted diet, as well as engaging in a high amount of exercise.[68] Finally, the cycle is affected more by a vegetarian diet compared to a non-vegetarian diet.[75]
Substance abuse
Studies investigating effects of the menstrual cycle on alcohol consumption have found mixed evidence.[76] However, some evidence suggests that individuals consume more alcohol during the luteal stage, especially if these individuals are heavy drinkers or have a family history of alcohol abuse.[70]
The level of substance abuse increases with PMS, mostly with addictive substances such as nicotine, tobacco and cocaine.[70] One theory behind this suggests this higher level of substance abuse is due to decreased self-control as a result of the higher metabolic demands during the luteal phase.[70]
Menstrual disorders
Infrequent or irregular ovulation is called oligoovulation.[77] The absence of ovulation is called anovulation. Normal menstrual flow can occur without ovulation preceding it: an anovulatory cycle. In some cycles, follicular development may start but not be completed; nevertheless, estrogens will be formed and stimulate the uterine lining. Anovulatory flow resulting from a very thick endometrium caused by prolonged, continued high estrogen levels is called estrogen breakthrough bleeding. Anovulatory bleeding triggered by a sudden drop in estrogen levels is called withdrawal bleeding.[78] Anovulatory cycles commonly occur before menopause (perimenopause) and in women with polycystic ovary syndrome.[79]
Very little flow (less than 10 ml) is called hypomenorrhea. Regular cycles with intervals of 21 days or fewer are polymenorrhea; frequent but irregular menstruation is known as metrorrhagia. Sudden heavy flows or amounts greater than 80 ml are termed menorrhagia.[80] Heavy menstruation that occurs frequently and irregularly is menometrorrhagia. The term for cycles with intervals exceeding 35 days is oligomenorrhea.[81] Amenorrhea refers to more than three[80] to six[81] months without menses (while not being pregnant) during a woman's reproductive years. The term for painful periods is Dysmenorrhea.
Cycles and phases


The menstrual cycle can be described by the ovarian or uterine cycle. The ovarian cycle describes changes that occur in the follicles of the ovary whereas the uterine cycle describes changes in the endometrial lining of the uterus. Both cycles can be divided into three phases. The ovarian cycle consists of the follicular phase, ovulation, and the luteal phase, whereas the uterine cycle consists of menstruation, proliferative phase, and secretory phase.[1]
Ovarian cycle
Follicular phase
The follicular phase is the first part of the ovarian cycle. During this phase, the ovarian follicles mature and get ready to release an egg.[1] The latter part of this phase overlaps with the proliferative phase of the uterine cycle.
Through the influence of a rise in follicle stimulating hormone (FSH) during the first days of the cycle, a few ovarian follicles are stimulated.[82] These follicles, which were present at birth[82] and have been developing for the better part of a year in a process known as folliculogenesis, compete with each other for dominance. Under the influence of several hormones, all but one of these follicles will stop growing, while one dominant follicle in the ovary will continue to maturity. The follicle that reaches maturity is called a tertiary or Graafian follicle, and it contains the ovum.[82]
Ovulation

Ovulation is the second phase of the ovarian cycle in which a mature egg is released from the ovarian follicles into the oviduct.[83] During the follicular phase, estradiol suppresses release of luteinizing hormone (LH) from the anterior pituitary gland. When the egg has nearly matured, levels of estradiol reach a threshold above which this effect is reversed and estrogen stimulates the production of a large amount of LH. This process, known as the LH surge, starts around day 12 of the average cycle and may last 48 hours.[84]
The exact mechanism of these opposite responses of LH levels to estradiol is not well understood.[85] In animals, a gonadotropin-releasing hormone (GnRH) surge has been shown to precede the LH surge, suggesting that estrogen's main effect is on the hypothalamus, which controls GnRH secretion.[85] This may be enabled by the presence of two different estrogen receptors in the hypothalamus: estrogen receptor alpha, which is responsible for the negative feedback estradiol-LH loop, and estrogen receptor beta, which is responsible for the positive estradiol-LH relationship.[86] However, in humans it has been shown that high levels of estradiol can provoke 32 increases in LH, even when GnRH levels and pulse frequencies are held constant,[85] suggesting that estrogen acts directly on the pituitary to provoke the LH surge.
The release of LH matures the egg and weakens the wall of the follicle in the ovary, causing the fully developed follicle to release its secondary oocyte.[82] If it is fertilized by a sperm, the secondary oocyte promptly matures into an ootid and then becomes a mature ovum. If it is not fertilized by a sperm, the secondary oocyte will degenerate. The mature ovum has a diameter of about 0.2 mm.[87]
Which of the two ovaries—left or right—ovulates appears essentially random; no known left and right co-ordination exists.[88] Occasionally, both ovaries will release an egg;[88] if both eggs are fertilized, the result is fraternal twins.[89]
After being released from the ovary, the egg is swept into the fallopian tube by the fimbria, which is a fringe of tissue at the end of each fallopian tube. After about a day, an unfertilized egg will disintegrate or dissolve in the fallopian tube.[82]
Fertilization by a spermatozoon, when it occurs, usually takes place in the ampulla, the widest section of the fallopian tubes. A fertilized egg immediately begins the process of embryogenesis, or development. The developing embryo takes about three days to reach the uterus and another three days to implant into the endometrium.[82] It has usually reached the blastocyst stage at the time of implantation.
In some women, ovulation features a characteristic pain called mittelschmerz (German term meaning middle pain).[90] The sudden change in hormones at the time of ovulation sometimes also causes light mid-cycle blood flow.[91]
Luteal phase
The luteal phase is the final phase of the ovarian cycle and it corresponds to the secretory phase of the uterine cycle. During the luteal phase, the pituitary hormones FSH and LH cause the remaining parts of the dominant follicle to transform into the corpus luteum, which produces progesterone. The increased progesterone in the adrenals starts to induce the production of estrogen. The hormones produced by the corpus luteum also suppress production of the FSH and LH that the corpus luteum needs to maintain itself. Consequently, the level of FSH and LH fall quickly over time, and the corpus luteum subsequently atrophies.[82] Falling levels of progesterone trigger menstruation and the beginning of the next cycle. From the time of ovulation until progesterone withdrawal has caused menstruation to begin, the process typically takes about two weeks, with 14 days considered normal. For an individual woman, the follicular phase often varies in length from cycle to cycle; by contrast, the length of her luteal phase will be fairly consistent from cycle to cycle.[92]
The loss of the corpus luteum is prevented by fertilization of the egg. The syncytiotrophoblast, which is the outer layer of the resulting embryo-containing structure (the blastocyst) and later also becomes the outer layer of the placenta, produces human chorionic gonadotropin (hCG), which is very similar to LH and which preserves the corpus luteum. The corpus luteum can then continue to secrete progesterone to maintain the new pregnancy. Most pregnancy tests look for the presence of hCG.[82]
Uterine cycle
The uterine cycle has three phases: menses, proliferative, secretory.[93]
Menstruation
Menstruation (also called menstrual bleeding, menses, catamenia or a period) is the first phase of the uterine cycle. The flow of menses normally serves as a sign that a woman has not become pregnant. (However, this cannot be taken as certainty, as a number of factors can cause bleeding during pregnancy; some factors are specific to early pregnancy, and some can cause heavy flow.)[94][95][96]
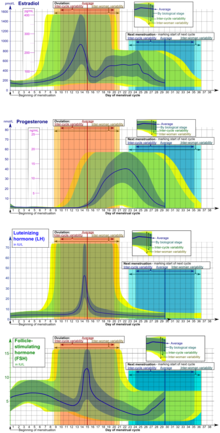
Eumenorrhea denotes normal, regular menstruation that lasts for a few days (usually 3 to 5 days, but anywhere from 2 to 7 days is considered normal).[90][97] The average blood loss during menstruation is 35 milliliters with 10–80 ml considered normal.[98] Women who experience Menorrhagia are more susceptible to iron deficiency than the average person.[99] An enzyme called plasmin inhibits clotting in the menstrual fluid.[100]
Painful cramping in the abdomen, back, or upper thighs is common during the first few days of menstruation. Severe uterine pain during menstruation is known as dysmenorrhea, and it is most common among adolescents and younger women (affecting about 67.2% of adolescent females).[101] When menstruation begins, symptoms of premenstrual syndrome (PMS) such as breast tenderness and irritability generally decrease.[90] Sanitary products include pads and tampons, and are essential items for use during menstruation.
Proliferative phase
The proliferative phase is the second phase of the uterine cycle when estrogen causes the lining of the uterus to grow, or proliferate, during this time.[82] As they mature, the ovarian follicles secrete increasing amounts of estradiol, and estrogen. The estrogens initiate the formation of a new layer of endometrium in the uterus, histologically identified as the proliferative endometrium. The estrogen also stimulates crypts in the cervix to produce cervical mucus, which causes vaginal discharge regardless of arousal, and can be tracked by women practicing fertility awareness.[102]
Secretory phase
The secretory phase is the final phase of the uterine cycle and it corresponds to the luteal phase of the ovarian cycle. During the secretory phase, the corpus luteum produces progesterone, which plays a vital role in making the endometrium receptive to implantation of the blastocyst and supportive of the early pregnancy, by increasing blood flow and uterine secretions and reducing the contractility of the smooth muscle in the uterus;[103] it also has the side effect of raising the woman's basal body temperature.[104]
Ovulation suppression
Birth control

While some forms of birth control do not affect the menstrual cycle, hormonal contraceptives work by disrupting it. Progestogen negative feedback decreases the pulse frequency of gonadotropin-releasing hormone (GnRH) release by the hypothalamus, which decreases the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary. Decreased levels of FSH inhibit follicular development, preventing an increase in estradiol levels. Progestogen negative feedback and the lack of estrogen positive feedback on LH release prevent a mid-cycle LH surge. Inhibition of follicular development and the absence of a LH surge prevent ovulation.[105][106][107]
The degree of ovulation suppression in progestogen-only contraceptives depends on the progestogen activity and dose. Low dose progestogen-only contraceptives—traditional progestogen only pills, subdermal implants Norplant and Jadelle, and intrauterine system Mirena—inhibit ovulation in about 50% of cycles and rely mainly on other effects, such as thickening of cervical mucus, for their contraceptive effectiveness.[108] Intermediate dose progestogen-only contraceptives—the progestogen-only pill Cerazette and the subdermal implant Nexplanon—allow some follicular development but more consistently inhibit ovulation in 97–99% of cycles. The same cervical mucus changes occur as with very low-dose progestogens. High-dose, progestogen-only contraceptives—the injectables Depo-Provera and Noristerat—completely inhibit follicular development and ovulation.[108]
Combined hormonal contraceptives include both an estrogen and a progestogen. Estrogen negative feedback on the anterior pituitary greatly decreases the release of FSH, which makes combined hormonal contraceptives more effective at inhibiting follicular development and preventing ovulation. Estrogen also reduces the incidence of irregular breakthrough bleeding.[105][106][107] Several combined hormonal contraceptives—the pill, NuvaRing, and the contraceptive patch—are usually used in a way that causes regular withdrawal bleeding. In a normal cycle, menstruation occurs when estrogen and progesterone levels drop rapidly.[104] Temporarily discontinuing use of combined hormonal contraceptives (a placebo week, not using patch or ring for a week) has a similar effect of causing the uterine lining to shed. If withdrawal bleeding is not desired, combined hormonal contraceptives may be taken continuously, although this increases the risk of breakthrough bleeding.
Breastfeeding
Breastfeeding causes negative feedback to occur on pulse secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH). Depending on the strength of the negative feedback, breastfeeding women may experience complete suppression of follicular development, but no ovulation, or normal menstrual cycle may resume.[109] Suppression of ovulation is more likely when suckling occurs more frequently.[110] The production of prolactin in response to suckling is important to maintaining lactational amenorrhea.[111] On average, women who are fully breastfeeding whose infants suckle frequently experience a return of menstruation at fourteen and a half months postpartum. There is a wide range of response among individual breastfeeding women, however, with some experiencing return of menstruation at two months and others remaining amenorrheic for up to 42 months postpartum.[112]
Society and culture
Etymological
The word "menstruation" is etymologically related to "moon". The terms "menstruation" and "menses" are derived from the Latin mensis (month), which in turn relates to the Greek mene (moon) and to the roots of the English words month and moon.[113]
The Moon
Even though the average length of the human menstrual cycle is similar to that of the lunar cycle, in modern humans there is no relation between the two.[114] The relationship is believed to be a coincidence.[115][116] Light exposure does not appear to affect the menstrual cycle in humans.[11] A meta-analysis of studies from 1996 showed no correlation between the human menstrual cycle and the lunar cycle[117], nor did data analysed by period-tracking app Clue, submitted by 1.5m women, of 7.5m menstrual cycles[118].
Dogon villagers did not have electric lighting and spent most nights outdoors, talking and sleeping, so they were apparently an ideal population for detecting a lunar influence; none was found.[119]
Work
In a number of countries, mainly in Asia, legislation or corporate practice has introduced formal menstrual leave to provide women with either paid or unpaid leave of absence from their employment while they are menstruating.[120] Countries with policies include Japan, Taiwan, Indonesia, and South Korea.[120] The practice is controversial due to concerns that it bolsters the perception of women as weak, inefficient workers,[120] as well as concerns that it is unfair to men.[121][122]
See also
References
- ^ a b c d e Silverthorn, Dee Unglaub (2013). Human Physiology: An Integrated Approach (6th ed.). Glenview, IL: Pearson Education. pp. 850–890. ISBN 978-0-321-75007-5.
- ^ Sherwood, Laurelee (2013). Human Physiology: From Cells to Systems (8th ed.). Belmont, California: Cengage. pp. 735–794. ISBN 978-1-111-57743-8.
- ^ a b Biggs, WS; Demuth, RH (15 October 2011). "Premenstrual syndrome and premenstrual dysphoric disorder". American Family Physician. 84 (8): 918–24. PMID 22010771.
- ^ "Premenstrual syndrome (PMS) fact sheet". Office on Women's Health, USA. 23 December 2014. Archived from the original on 28 June 2015. Retrieved 23 June 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Women's Gynecologic Health. Jones & Bartlett Publishers. 2011. p. 94. ISBN 9780763756376.
- ^ a b c d e "Menstruation and the menstrual cycle fact sheet". Office of Women's Health, USA. 23 December 2014. Archived from the original on 26 June 2015. Retrieved 25 June 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Chiazze L, Brayer FT, Macisco JJ, Parker MP, Duffy BJ (February 1968). "The length and variability of the human menstrual cycle". JAMA. 203 (6): 377–80. doi:10.1001/jama.1968.03140060001001. PMID 5694118.
- ^ a b American Academy of Pediatrics Committee on, Adolescence; American College of Obstetricians and Gynecologists Committee on Adolescent Health, Care; Diaz, A; Laufer, MR; Breech, LL (November 2006). "Menstruation in girls and adolescents: using the menstrual cycle as a vital sign". Pediatrics. 118 (5): 2245–50. doi:10.1542/peds.2006-2481. PMID 17079600.
- ^ "Menopause: Overview". NIH. 28 June 2013. Retrieved 8 March 2015.
- ^ Klump KL, Keel PK, Racine SE, Burt SA, Burt AS, Neale M, Sisk CL, Boker S, Hu JY (February 2013). "The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle". J Abnorm Psychol. 122 (1): 131–7. doi:10.1037/a0029524. PMC 3570621. PMID 22889242.
- ^ a b Kristin H. Lopez (2013). Human Reproductive Biology. Academic Press. p. 53. ISBN 9780123821850.
- ^ Karapanou, O.; Papadimitriou, A. (2010). "Determinants of menarche". Reprod Biol Endocrinol. 8: 115. doi:10.1186/1477-7827-8-115. PMC 2958977. PMID 20920296.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)CS1 maint: unflagged free DOI (link) - ^ Anderson SE, Dallal GE, Must A (April 2003). "Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart". Pediatrics. 111 (4 Pt 1): 844–50. doi:10.1542/peds.111.4.844. PMID 12671122.
- ^ Al-Sahab B, Ardern CI, Hamadeh MJ, Tamim H (2010). "Age at menarche in Canada: results from the National Longitudinal Survey of Children & Youth". BMC Public Health. 10: 736. doi:10.1186/1471-2458-10-736. PMC 3001737. PMID 21110899.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hamilton-Fairley, Diana (2004) [1999]. Lecture notes on obstetrics and gynaecology (PDF) (2nd ed.). Blackwell. p. 29. ISBN 978-1-4051-2066-1.
- ^ Macgússon TE (May 1978). "Age at menarche in Iceland". Am. J. Phys. Anthropol. 48 (4): 511–4. doi:10.1002/ajpa.1330480410. PMID 655271.
- ^ "At what age does a girl get her first period?". National Women's Health Information Center. Archived from the original on 23 November 2011. Retrieved 20 November 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Clinical topic — Menopause". NHS. Archived from the original on 7 July 2009. Retrieved 2 November 2009.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ Beyene, Yewoubdar (1989). From Menarche to Menopause: Reproductive Lives of Peasant Women in Two Cultures. Albany, NY: State University of New York Press. ISBN 978-0-88706-866-9.
- ^ Shuman, Tracy (February 2006). "Your Guide to Menopause". WebMD. Retrieved 16 December 2006.
- ^ Kippley, John; Kippley, Sheila (1996). The Art of Natural Family Planning (4th ed.). Cincinnati: The Couple to Couple League. p. 92. ISBN 978-0-926412-13-2.
- ^ Lenton EA, Landgren BM, Sexton L (July 1984). "Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase". Br J Obstet Gynaecol. 91 (7): 685–9. doi:10.1111/j.1471-0528.1984.tb04831.x. PMID 6743610.
- ^ a b Lenton EA, Landgren BM, Sexton L, Harper R (July 1984). "Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age". Br J Obstet Gynaecol. 91 (7): 681–4. doi:10.1111/j.1471-0528.1984.tb04830.x. PMID 6743609.
- ^ "Migraine and Estrogen Officially Linked". The Daily Headache. 19 April 2006. Retrieved 19 October 2012.
- ^ a b Herzog AG (March 2008). "Catamenial epilepsy: definition, prevalence pathophysiology and treatment". Seizure. 17 (2): 151–9. doi:10.1016/j.seizure.2007.11.014. PMID 18164632.
- ^ Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M (September 2004). "Frequency of catamenial seizure exacerbation in women with localization-related epilepsy". Annals of Neurology. 56 (3): 431–4. doi:10.1002/ana.20214. PMID 15349872.
- ^ Herzog AG, Klein P, Ransil BJ (October 1997). "Three patterns of catamenial epilepsy". Epilepsia. 38 (10): 1082–8. doi:10.1111/j.1528-1157.1997.tb01197.x. PMID 9579954.
- ^ Scharfman HE, MacLusky NJ (September 2006). "The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female". Epilepsia. 47 (9): 1423–40. doi:10.1111/j.1528-1167.2006.00672.x. PMC 1924802. PMID 16981857.
- ^ "Menstrual cycle". epilepsy.com. Archived from the original on 15 October 2012. Retrieved 19 October 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Baca-García E, Diaz-Sastre C, Ceverino A, Saiz-Ruiz J, Diaz FJ, de Leon J (2003). "Association between the menses and suicide attempts: a replication study". Psychosom Med. 65 (2): 237–44. CiteSeerX 10.1.1.494.4039. doi:10.1097/01.PSY.0000058375.50240.F6. PMID 12651991.
- ^ Maguire JL, Stell BM, Rafizadeh M, Mody I (June 2005). "Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety". Nat. Neurosci. 8 (6): 797–804. doi:10.1038/nn1469. PMID 15895085.
- ^ Doufas AG, Mastorakos G (2000). "The hypothalamic-pituitary-thyroid axis and the female reproductive system". Annals of the New York Academy of Sciences. 900: 65–76. doi:10.1111/j.1749-6632.2000.tb06217.x. PMID 10818393.
- ^ Krejza J, Nowacka A, Szylak A, Bilello M, Melhem LY (July 2004). "Variability of thyroid blood flow Doppler parameters in healthy women". Ultrasound Med Biol. 30 (7): 867–76. doi:10.1016/j.ultrasmedbio.2004.05.008. PMID 15313319.
- ^ Stern K, McClintock MK (March 1998). "Regulation of ovulation by human pheromones". Nature. 392 (6672): 177–9. doi:10.1038/32408. PMID 9515961.
- ^ Adams, Cecil (20 December 2002). "Does menstrual synchrony really exist?". The Straight Dope. The Chicago Reader. Retrieved 10 January 2007.
- ^ Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett W, Georgoulis T, Hewett TE, Johnson R, Krosshaug T, Mandelbaum B, Micheli L, Myklebust G, Roos E, Roos H, Schamasch P, Shultz S, Werner S, Wojtys E, Engebretsen L (June 2008). "Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement". Br J Sports Med. 42 (6): 394–412. doi:10.1136/bjsm.2008.048934. PMC 3920910. PMID 18539658.
- ^ Dunson, D.B.; Baird, D.D.; Wilcox, A.J.; Weinberg, C.R. (1999). "Day-specific probabilities of clinical pregnancy based on two studies with imperfect measures of ovulation". Human Reproduction. 14 (7): 1835–1839. doi:10.1093/humrep/14.7.1835. ISSN 1460-2350.
- ^ Weschler (2002), pp.242,374
- ^ MedlinePlus Encyclopedia: LH urine test (home test)
- ^ a b "Medical Eligibility Criteria for Contraceptive Use:Fertility awareness-based methods". Third edition. World Health Organization. 2004. Retrieved 29 April 2008.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Weschler (2002), p.52
- ^ Leridon H (July 2004). "Can assisted reproduction technology compensate for the natural decline in fertility with age? A model assessment". Hum. Reprod. 19 (7): 1548–53. doi:10.1093/humrep/deh304. PMID 15205397.
- ^ Krock, Lexi (October 2001). "Fertility Throughout Life". 18 Ways to Make a Baby. NOVA Online. Retrieved 24 December 2006. Haines, Cynthiac (January 2006). "Your Guide to the Female Reproductive System". The Cleveland Clinic Women's Health Center. WebMD. Retrieved 24 December 2006.
- ^ a b c Brodin T, Bergh T, Berglund L, Hadziosmanovic N, Holte J (2008). "Menstrual cycle length is an age-independent marker of female fertility: Results from 6271 treatment cycles of in vitro fertilization". Fertility and Sterility. 90 (5): 1656–1661. doi:10.1016/j.fertnstert.2007.09.036. PMID 18155201.
- ^ Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR (January 1998). "Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome". N. Engl. J. Med. 338 (4): 209–16. doi:10.1056/NEJM199801223380401. PMID 9435325.
- ^ Derntl B, Hack RL, Kryspin-Exner I, Habel U (January 2013). "Association of menstrual cycle phase with the core components of empathy". Horm Behav. 63 (1): 97–104. doi:10.1016/j.yhbeh.2012.10.009. PMC 3549494. PMID 23098806.
- ^ Schwartz DH, Romans SE, Meiyappan S, De Souza MJ, Einstein G (September 2012). "The role of ovarian steroid hormones in mood". Horm Behav. 62 (4): 448–54. doi:10.1016/j.yhbeh.2012.08.001. PMID 22902271.
- ^ Pearson R, Lewis MB (March 2005). "Fear recognition across the menstrual cycle". Horm Behav. 47 (3): 267–71. doi:10.1016/j.yhbeh.2004.11.003. PMID 15708754.
- ^ Levay, Simon; Baldwin, Janice; Baldwin, John (2015). "Women's Bodies". Discovering Human Sexuality. Massachusetts: Sinauer Associtates, Inc. p. 44. ISBN 9781605352756.
- ^ Thornhill, Randy; Gangestad, Steven W (2008). "Background and Overview of the Book". The Evolutionary Biology of Human Female Sexuality. New York: Oxford University Press. p. 12. ISBN 9780195340990.
- ^ Gallino, Lucila; Carrillo, Facundo; Cecchi, Guillermo A. (19 February 2019). "Differential 28-days cyclic modulation of affective intensity in female and male participants via social media". Frontiers in Integrative Neuroscience. 13: 5. doi:10.3389/fnint.2019.00005.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Gildersleeve, Kelly; Haselton, Martie G.; Fales, Melissa R. (2014). "Meta-analyses and p-curves support robust cycle shifts in women's mate preferences: Reply to Wood and Carden (2014) and Harris, Pashler, and Mickes (2014)". Psychological Bulletin. 140 (5): 1272–1280. doi:10.1037/a0037714. PMID 25180805.
- ^ Gildersleeve, Kelly; DeBruine, Lisa; Haselton, Martie G.; Frederick, David A.; Penton-Voak, Ian S.; Jones, Benedict C.; Perrett, David I. (11 April 2013). "Shifts in Women's Mate Preferences Across the Ovulatory Cycle: A Critique of Harris (2011) and Harris (2012)". Sex Roles. 69 (9–10): 516–524. doi:10.1007/s11199-013-0273-4. ISSN 0360-0025.
- ^ a b c d Levay, Simon; Valente, Sharon M (2006). "Sexual Attraction and Arousal". Human Sexuality. Massachusetts: Sinauer Associates Inc. pp. 217–253. ISBN 978-0878934652.
- ^ a b c d Thornhill, Randy; Gangestad, Steven W (2008). "Women's Estrus, Pair Bonding and Extra-Pair Sex". The Evolutionary Biology of Human Female Sexuality. New York: Oxford University Press. pp. 246–256. ISBN 9780195340990.
- ^ DeBruine, L; Jones, BC; Frederick, DA; Haselton, MG; Penton-Voak, IS; Perrett, DI (10 December 2010). "Evidence for menstrual cycle shifts in women's preferences for masculinity: a response to Harris (in press) "Menstrual cycle and facial preferences reconsidered"". Evolutionary Psychology : An International Journal of Evolutionary Approaches to Psychology and Behavior. 8 (4): 768–75. doi:10.1177/147470491000800416. PMID 22947833.
- ^ Thornhill, Randy; Gangestad, Steven W (2008). "Coevolutionary Processes". The Evolutionary Biology of Human Female Sexuality. New York: Oxford University Press. pp. 290–321. ISBN 9780195340990.
- ^ Levay, Simon; Baldwin, Janice; Baldwin, John (2015). "Sex and Evolution". Discovering Human Sexuality. Massachusetts: Sinauer Associates, Inc. p. 565. ISBN 9781605352756.
- ^ a b c d e f g h i j k l m n Thornhill, Randy; Gangestad, Steven W (2008). "Women's Estrus". The Evolutionary Biology of Human Female Sexuality. New York: Oxford University Press. pp. 207–234. ISBN 9780195340990.
- ^ a b c Levay, Simon; Baldwin, Janice; Baldwin, John (2015). "Attraction, Arousal, and Response". Discovering Human Sexuality. Sunderland, Massachusetts U.S.A: Sinauer Associates, Inc. p. 135. ISBN 9781605352756.
- ^ a b c Pipitone, R. Nathan; Gallup, Gordon G. (1 July 2008). "Women's voice attractiveness varies across the menstrual cycle". Evolution and Human Behavior. 29 (4): 268–274. doi:10.1016/j.evolhumbehav.2008.02.001. ISSN 1090-5138.
- ^ a b c d Thornhill, Randy; Gangestad, Steven W (2008). "Concealed Fertility". The Evolutionary Biology of Human Female Sexuality. New York: Oxford University Press. pp. 266–290. ISBN 9780195340990.
- ^ Gangestad, Steven W.; Thornhill, Randy (22 May 1998). "Menstrual cycle variation in women's preferences for the scent of symmetrical men". Proceedings of the Royal Society of London B: Biological Sciences. 265 (1399): 927–933. doi:10.1098/rspb.1998.0380. ISSN 0962-8452. PMC 1689051. PMID 9633114.
- ^ a b DeBruine, Lisa; Jones, Benedict C.; Frederick, David A.; Haselton, Martie G.; Penton-Voak, Ian S.; Perrett, David I. (1 January 2010). "Evidence for menstrual cycle shifts in women's preferences for masculinity: a response to Harris (in press) "Menstrual cycle and facial preferences reconsidered"". Evolutionary Psychology: An International Journal of Evolutionary Approaches to Psychology and Behavior. 8 (4): 768–775. doi:10.1177/147470491000800416. ISSN 1474-7049. PMID 22947833.
- ^ a b Jones, Benedict C.; DeBruine, Lisa M.; Perrett, David I.; Little, Anthony C.; Feinberg, David R.; Smith, Miriam J. Law (12 January 2008). "Effects of Menstrual Cycle Phase on Face Preferences". Archives of Sexual Behavior. 37 (1): 78–84. doi:10.1007/s10508-007-9268-y. ISSN 0004-0002. PMID 18193349.
- ^ Gildersleeve, Kelly; Haselton, Martie G.; Fales, Melissa R. (2014). "Do women's mate preferences change across the ovulatory cycle? A meta-analytic review". Psychological Bulletin. 140 (5): 1205–1259. doi:10.1037/a0035438. PMID 24564172.
- ^ a b c Dye, L.; Blundell, J. E. (1 June 1997). "Menstrual cycle and appetite control: implications for weight regulation". Human Reproduction. 12 (6): 1142–1151. doi:10.1093/humrep/12.6.1142. ISSN 0268-1161. PMID 9221991.
- ^ a b c d e f Buffenstein, Rochelle; Poppitt, Sally D.; McDevitt, Regina M.; Prentice, Andrew M. (1 December 1995). "Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research". Physiology & Behavior. 58 (6): 1067–1077. doi:10.1016/0031-9384(95)02003-9.
- ^ a b c Davidsen, L.; Vistisen, B.; Astrup, A. (7 August 2007). "Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts". International Journal of Obesity. 31 (12): 1777–1785. doi:10.1038/sj.ijo.0803699. ISSN 0307-0565. PMID 17684511.
- ^ a b c d Gailliot, Matthew T.; Hildebrandt, Britny; Eckel, Lisa A.; Baumeister, Roy F. (2010). "A theory of limited metabolic energy and premenstrual syndrome symptoms: Increased metabolic demands during the luteal phase divert metabolic resources from and impair self-control". Review of General Psychology. 14 (3): 269–282. doi:10.1037/a0018525.
- ^ Wurtman, Judith J.; Brzezinski, Amnon; Wurtman, Richard J.; Laferrere, Blandine (1989). "Effect of nutrient intake on premenstrual depression". American Journal of Obstetrics and Gynecology. 161 (5): 1228–1234. doi:10.1016/0002-9378(89)90671-6.
- ^ Klump, K. L.; Keel, P. K.; Culbert, K. M.; Edler, C. (1 December 2008). "Ovarian hormones and binge eating: exploring associations in community samples". Psychological Medicine. 38 (12): 1749–1757. doi:10.1017/S0033291708002997. ISSN 1469-8978. PMC 2885896. PMID 18307829.
- ^ Blundell, J. E. (1 December 1984). "Serotonin and appetite". Neuropharmacology. 23 (12, Part 2): 1537–1551. doi:10.1016/0028-3908(84)90098-4.
- ^ Bethea, C. L.; Gundlah, C.; Mirkes, S. J. (1 January 2000). "Ovarian steroid action in the serotonin neural system of macaques". Novartis Foundation Symposium. 230: 112–130, discussion 130–133. ISSN 1528-2511. PMID 10965505.
- ^ Dye, L.; Blundell, J. E. (1 June 1997). "Menstrual cycle and appetite control: implications for weight regulation". Human Reproduction. 12 (6): 1142–1151. doi:10.1093/humrep/12.6.1142. ISSN 0268-1161. PMID 9221991.
- ^ Carroll, Haley A.; Lustyk, M. Kathleen B.; Larimer, Mary E. (21 August 2015). "The relationship between alcohol consumption and menstrual cycle: a review of the literature". Archives of Women's Mental Health. 18 (6): 773–781. doi:10.1007/s00737-015-0568-2. ISSN 1434-1816. PMC 4859868. PMID 26293593.
- ^ Galan, Nicole (16 April 2008). "Oligoovulation". about.com. Retrieved 12 October 2008.
- ^ Weschler (2002), p.107
- ^ Anovulation at eMedicine
- ^ a b Menstruation Disorders at eMedicine
- ^ a b Oriel KA, Schrager S (October 1999). "Abnormal uterine bleeding". Am Fam Physician. 60 (5): 1371–80, discussion 1381–2. PMID 10524483.
- ^ a b c d e f g h i Losos, Jonathan B.; Raven, Peter H.; Johnson, George B.; Singer, Susan R. (2002). Biology. New York: McGraw-Hill. pp. 1207–09. ISBN 978-0-07-303120-0.
- ^ Ovulation Test at Duke Fertility Center. Retrieved 2 July 2011
- ^ "Ovulation Calendar". Pregnology.
- ^ a b c Comprehensive gynecology. St. Louis: Elsevier Mosby. 2013. ISBN 978-0-323-06986-1. Retrieved 5 April 2012.
{{cite book}}: Unknown parameter|authors=ignored (help) - ^ Hu L, Gustofson RL, Feng H, Leung PK, Mores N, Krsmanovic LZ, Catt KJ (October 2008). "Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons". Mol. Endocrinol. 22 (10): 2250–9. doi:10.1210/me.2008-0192. PMC 2582533. PMID 18701637.
- ^ Gray, Henry David (1918). "The Ovum". Anatomy of the human body. Philadelphia: Lea & Febiger – via Bartleby.com.
- ^ a b Ecochard R, Gougeon A (April 2000). "Side of ovulation and cycle characteristics in normally fertile women". Hum. Reprod. 15 (4): 752–5. doi:10.1093/humrep/15.4.752. PMID 10739814.
- ^ "Multiple Pregnancy: Twins or More — Topic Overview". WebMD Medical Reference from Healthwise. 24 July 2007. Retrieved 5 October 2008.
- ^ a b c John M Goldenring (1 February 2007). "All About Menstruation". WebMD. Retrieved 5 October 2008.
- ^ Weschler (2002), p.65
- ^ Weschler (2002), p.47
- ^ "Chapter 81 Female Physiology Before Pregnancy and Female Hormones". Textbook of Medical Physiology (11th ed.). Elsevier Saunders. 2006. pp. 1018ff. ISBN 9780721602400.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ Greenfield, Marjorie (17 September 2001). "Subchorionic Hematoma in Early Pregnancy". Ask Our Experts. DrSpock.com. Archived from the original on 15 September 2008. Retrieved 21 September 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Anderson-Berry, Ann L; Terence Zach (10 December 2007). "Vanishing Twin Syndrome". Emedicine.com. Retrieved 21 September 2008.
- ^ Ko, Patrick; Young Yoon (23 August 2007). "Placenta Previa". Emedicine.com. Retrieved 21 September 2008.
- ^ "Menstruation and the Menstrual Cycle". Womenshealth.gov. 23 December 2014. Archived from the original on 26 June 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ David L Healy (24 November 2004). "Menorrhagia Heavy Periods — Current Issues". Monash University. ABN 12 377 614 012. Archived from the original on 16 October 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ (October 2005). "Impact of menstrual blood loss and diet on iron deficiency among women in the UK". Br. J. Nutr. 94 (4): 557–64. doi:10.1079/BJN20051493. PMID 16197581.
- ^ Shiraishi M (August 1962). "Studies on identification of menstrual blood stain by fibrin-plate method. I. A study on the incoagulability of menstrual blood". Acta Med Okayama. 16: 192–200. PMID 13977381. Archived from the original on 9 August 2014.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Sharma P, Malhotra C, Taneja DK, Saha R (February 2008). "Problems related to menstruation amongst adolescent girls". Indian J Pediatr. 75 (2): 125–9. doi:10.1007/s12098-008-0018-5. PMID 18334791.
- ^ Weschler, Toni (2002). Taking Charge of Your Fertility (Revised ed.). New York: HarperCollins. pp. 359–361. ISBN 978-0-06-093764-5.
- ^ Lombardi, Julian (1998). Comparative Vertebrate Reproduction. Springer. p. 184. ISBN 9780792383369.
- ^ a b Weschler (2002), pp.361-2
- ^ a b Trussell, James (2007). "Contraceptive Efficacy". In Hatcher, Robert A.; et al. (eds.). Contraceptive Technology (19th rev. ed.). New York: Ardent Media. ISBN 978-0-9664902-0-6.
{{cite book}}: External link in|chapterurl=|chapterurl=ignored (|chapter-url=suggested) (help) - ^ a b Speroff, Leon; Darney, Philip D. (2005). "Oral Contraception". A Clinical Guide for Contraception (4th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 21–138. ISBN 978-0-7817-6488-9.
- ^ a b Brunton, Laurence L.; Lazo, John S.; Parker, Keith, eds. (2005). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. pp. 1541–71. ISBN 978-0-07-142280-2.
- ^ a b Glasier, Anna (2006). "Contraception". In DeGroot, Leslie J.; Jameson, J. Larry (eds.). Endocrinology (5th ed.). Philadelphia: Elsevier Saunders. pp. 3000–1. ISBN 978-0-7216-0376-6.
- ^ McNeilly AS (2001). "Lactational control of reproduction". Reprod. Fertil. Dev. 13 (7–8): 583–90. doi:10.1071/RD01056. PMID 11999309.
- ^ Kippley, John; Sheila Kippley (1996). The Art of Natural Family Planning (4th ed.). Cincinnati, OH: The Couple to Couple League. p. 347. ISBN 978-0-926412-13-2.
- ^ Stallings JF, Worthman CM, Panter-Brick C, Coates RJ (February 1996). "Prolactin response to suckling and maintenance of postpartum amenorrhea among intensively breastfeeding Nepali women". Endocr. Res. 22 (1): 1–28. doi:10.3109/07435809609030495. PMID 8690004.
- ^ "Breastfeeding: Does It Really Space Babies?". The Couple to Couple League International. Internet Archive. 17 January 2008. Archived from the original on 17 January 2008. Retrieved 21 September 2008., which cites:
- Kippley SK, Kippley JF (November–December 1972). "The relation between breastfeeding and amenorrhea". Journal of Obstetric, Gynecologic, & Neonatal Nursing. 1 (4): 15–21. doi:10.1111/j.1552-6909.1972.tb00558.x. PMID 4485271.
- Sheila Kippley (November–December 1986). "Breastfeeding survey results similar to 1971 study". The CCL News. 13 (3): 10. and (January–February 1987) 13 (4): 5.
- ^ Allen, Kevin (2007). The Reluctant Hypothesis: A History of Discourse Surrounding the Lunar Phase Method of Regulating Conception. Lacuna Press. p. 239. ISBN 978-0-9510974-2-7.
- ^ Vertebrate Endocrinology (5 ed.). Academic Press. 2013. p. 361. ISBN 9780123964656.
- ^ William A. Gutsch (1997). 1001 things everyone should know about the universe (1st ed.). New York: Doubleday. p. 57. ISBN 9780385482233.
- ^ Barash, David P.; Lipton, Judith Eve (2009). "Synchrony and Its Discontents". How women got their curves and other just-so stories evolutionary enigmas ([Online-Ausg.]. ed.). New York: Columbia University Press. ISBN 9780231518390.
- ^ As cited by Adams, Cecil, "What's the link between the moon and menstruation?" (accessed 6 June 2006):
Abell, George O.; Barry Singer (1983). Science and the Paranormal: Probing the Existence of the Supernatural. Scribner Book Company. ISBN 978-0-684-17820-2. - ^ "The myth of moon phases and menstruation". Clue. 3 December 2018. Retrieved 3 December 2018.
- ^ Strassmann B. I. (1997). "The biology of menstruation in Homo sapiens: total lifetime menses, fecundity, and nonsynchrony in a natural fertility population". Current Anthropology. 38 (1): 123–9. doi:10.1086/204592. JSTOR 2744446.
- ^ a b c Emily Matchar (16 May 2014). "Should Paid 'Menstrual Leave' Be a Thing?". Retrieved 21 June 2015.
- ^ Price, Catherine (11 October 2006). "Should women get paid menstruation leave?". Salon. Retrieved 16 March 2016.
- ^ "Menstrual Leave: Delightful or Discriminatory? | Culture | Lip Magazine". lip magazine. Retrieved 16 March 2016.
External links
![]() Media related to Menstrual cycle at Wikimedia Commons
Media related to Menstrual cycle at Wikimedia Commons
