Chloroquine: Difference between revisions
→COVID-19: added RCT |
|||
| Line 240: | Line 240: | ||
On 27 March 2020, the FDA issued guidance, "do not use chloroquine phosphate intended for fish as treatment for COVID-19 in humans".<ref>{{cite web | title=FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans | website=U.S. [[Food and Drug Administration]] (FDA) | date=27 March 2020 | url=http://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-chloroquine-phosphate-intended-fish-treatment-covid-19-humans | access-date=1 April 2020}}</ref> |
On 27 March 2020, the FDA issued guidance, "do not use chloroquine phosphate intended for fish as treatment for COVID-19 in humans".<ref>{{cite web | title=FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans | website=U.S. [[Food and Drug Administration]] (FDA) | date=27 March 2020 | url=http://www.fda.gov/animal-veterinary/product-safety-information/fda-letter-stakeholders-do-not-use-chloroquine-phosphate-intended-fish-treatment-covid-19-humans | access-date=1 April 2020}}</ref> |
||
On 1 April 2020, the [[European Medicines Agency]] (EMA) issued guidance that chloroquine and hydroxychloroquine are only to be used in clinical trials or emergency use programs.<ref>{{cite web | title=COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes | website=[[European Medicines Agency]] (EMA) | date=1 April 2020 | url=https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes | access-date=2 April 2020}}</ref> |
On 1 April 2020, the [[European Medicines Agency]] (EMA) issued guidance that chloroquine and hydroxychloroquine are only to be used in clinical trials or emergency use programs.<ref>{{cite web | title=COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes | website=[[European Medicines Agency]] (EMA) | date=1 April 2020 | url=https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes | access-date=2 April 2020}}</ref>. |
||
On the same date, initial results from a [[randomised controlled trial]] conducted in China that compared Chloroquine to [[Lopinavir/ritonavir]] were published. The study found evidence that Chloroquine was slightly more effective than Lopinavir/ritonavir at treating COVID-19 patients who met the study's enrollment criteria, and noted that patients treated with Chloroquine recovered better and regained their pulmonary function more quickly than patients treated with Lopinavir/ritonavir.<ref>{{cite journal|last1=Huang|first1=Mingxing|last2=Tang|first2=Tiantian|last3=Pang|first3=Pengfei|last4=Li|first4=Man|last5=Ma|first5=Ruolan|last6=Lu|first6=Jiahui|last7=Shu|first7=Jingxian|last8=You|first8=Yingying|last9=Chen|first9=Binghui|title=Treating COVID-19 with Chloroquine|date=1 April 2020|access-date=3 April 2020|url=https://academic.oup.com/jmcb/advance-article/doi/10.1093/jmcb/mjaa014/5814655 |journal=Journal of Molecular Cell Biology |doi=10.1093/jmcb/mjaa014 |pmid=32236562 }}</ref> |
|||
=== Other viruses === |
=== Other viruses === |
||
Revision as of 08:43, 3 April 2020
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈklɔːrəkwiːn/ |
| Trade names | Aralen, other |
| Other names | Chloroquine phosphate |
| AHFS/Drugs.com | Monograph |
| License data |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 1-2 months |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.175 |
| Chemical and physical data | |
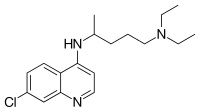
| Formula | C18H26ClN3 |
| Molar mass | 319.872 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Chloroquine is a medication primarily used to prevent and treat malaria in areas where malaria remains sensitive to its effects.[1] Certain types of malaria, resistant strains, and complicated cases typically require different or additional medication.[1] Chloroquine is also occasionally used for amebiasis that is occurring outside the intestines, rheumatoid arthritis, and lupus erythematosus.[1] While it has not been formally studied in pregnancy, it appears safe.[1][2] It is also being studied to treat COVID-19 as of 2020.[3] It is taken by mouth.[1]
Common side effects include muscle problems, loss of appetite, diarrhea, and skin rash.[1] Serious side effects include problems with vision, muscle damage, seizures, and low blood cell levels.[1][4] Chloroquine is a member of the drug class 4-aminoquinoline.[1] As an antimalarial, it works against the asexual form of the malaria parasite in the stage of its life cycle within the red blood cell.[1] How it works in rheumatoid arthritis and lupus erythematosus is unclear.[1]
Chloroquine was discovered in 1934 by Hans Andersag.[5][6] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[7] It is available as a generic medication.[1] The wholesale cost in the developing world is about US$0.04.[8] In the United States, it costs about US$5.30 per dose.[1]
Medical uses
Malaria

♦ Elevated occurrence of chloroquine- or multi-resistant malaria
♦ Occurrence of chloroquine-resistant malaria
♦ No Plasmodium falciparum or chloroquine-resistance
♦ No malaria
Chloroquine has been used in the treatment and prevention of malaria from Plasmodium vivax, P. ovale, and P. malariae. It is generally not used for Plasmodium falciparum as there is widespread resistance to it.[10][11]
Chloroquine has been extensively used in mass drug administrations, which may have contributed to the emergence and spread of resistance. It is recommended to check if chloroquine is still effective in the region prior to using it.[12] In areas where resistance is present, other antimalarials, such as mefloquine or atovaquone, may be used instead. The Centers for Disease Control and Prevention recommend against treatment of malaria with chloroquine alone due to more effective combinations.[13]
Amebiasis
In treatment of amoebic liver abscess, chloroquine may be used instead of or in addition to other medications in the event of failure of improvement with metronidazole or another nitroimidazole within 5 days or intolerance to metronidazole or a nitroimidazole.[14]
Rheumatic disease
As it mildly suppresses the immune system, chloroquine is used in some autoimmune disorders, such as rheumatoid arthritis and lupus erythematosus.[1]
Side effects
Side effects include blurred vision, nausea, vomiting, abdominal cramps, headache, diarrhea, swelling legs/ankles, shortness of breath, pale lips/nails/skin, muscle weakness, easy bruising/bleeding, hearing and mental problems.[15][16]
- Unwanted/uncontrolled movements (including tongue and face twitching) [15]
- Deafness or tinnitus.[15]
- Nausea, vomiting, diarrhea, abdominal cramps[16]
- Headache.[15]
- Mental/mood changes (such as confusion, personality changes, unusual thoughts/behavior, depression, feeling being watched, hallucinating)[15][16]
- Signs of serious infection (such as high fever, severe chills, persistent sore throat)[15]
- Skin itchiness, skin color changes, hair loss, and skin rashes.[16][17]
- Chloroquine-induced itching is very common among black Africans (70%), but much less common in other races. It increases with age, and is so severe as to stop compliance with drug therapy. It is increased during malaria fever; its severity is correlated to the malaria parasite load in blood. Some evidence indicates it has a genetic basis and is related to chloroquine action with opiate receptors centrally or peripherally.[18]
- Unpleasant metallic taste
- This could be avoided by "taste-masked and controlled release" formulations such as multiple emulsions.[19]
- Chloroquine retinopathy
- Electrocardiographic changes[20]
- This manifests itself as either conduction disturbances (bundle-branch block, atrioventricular block) or Cardiomyopathy – often with hypertrophy, restrictive physiology, and congestive heart failure. The changes may be irreversible. Only two cases have been reported requiring heart transplantation, suggesting this particular risk is very low. Electron microscopy of cardiac biopsies show pathognomonic cytoplasmic inclusion bodies.
- Pancytopenia, aplastic anemia, reversible agranulocytosis, low blood platelets, neutropenia.[21]
Pregnancy
Chloroquine has not been shown to have any harmful effects on the fetus when used in the recommended doses for malarial prophylaxis.[22] Small amounts of chloroquine are excreted in the breast milk of lactating women. However, this drug can be safely prescribed to infants, the effects are not harmful. Studies with mice show that radioactively tagged chloroquine passed through the placenta rapidly and accumulated in the fetal eyes which remained present five months after the drug was cleared from the rest of the body.[21][23] Women who are pregnant or planning on getting pregnant are still advised against traveling to malaria-risk regions.[22]
Elderly
There is not enough evidence to determine whether chloroquine is safe to be given to people aged 65 and older. Since it is cleared by the kidneys, toxicity should be monitored carefully in people with poor kidney functions.[21]
Drug interactions
Chloroquine has a number of drug–drug interactions that might be of clinical concern:[citation needed]
- Ampicillin- levels may be reduced by chloroquine;[21]
- Antacids- may reduce absorption of chloroquine;[21]
- Cimetidine- may inhibit metabolism of chloroquine; increasing levels of chloroquine in the body;[21]
- Cyclosporine- levels may be increased by chloroquine;[21] and
- Mefloquine- may increase risk of convulsions.[21]
Overdose
Chloroquine is very dangerous in overdose. It is rapidly absorbed from the gut. In 1961, a published compilation of case reports contained accounts of three children who took overdoses and died within 2.5 hours of taking the drug. While the amount of the overdose was not stated, the therapeutic index for chloroquine is known to be small.[24] One of the children died after taking 0.75 or 1 gram, or twice a single therapeutic amount for children. Symptoms of overdose include headache, drowsiness, visual disturbances, nausea and vomiting, cardiovascular collapse, seizures, and sudden respiratory and cardiac arrest.[21]
An analog of chloroquine – hydroxychloroquine – has a long half-life (32–56 days) in blood and a large volume of distribution (580–815 L/kg).[25] The therapeutic, toxic and lethal ranges are usually considered to be 0.03 to 15 mg/l, 3.0 to 26 mg/l and 20 to 104 mg/l, respectively. However, nontoxic cases have been reported up to 39 mg/l, suggesting individual tolerance to this agent may be more variable than previously recognised.[25]
Pharmacology
This section needs additional citations for verification. (July 2015) |
Chloroquine's absorption of the drug is rapid.[citation needed] It is widely distributed in body tissues.[citation needed] Its protein binding is 55%.[clarification needed][citation needed] Its metabolism is partially hepatic, giving rise to its main metabolite, desethylchloroquine.[citation needed] Its excretion is ≥50% as unchanged drug in urine, where acidification of urine increases its elimination.[citation needed] It has a very high volume of distribution, as it diffuses into the body's adipose tissue.[citation needed]
Accumulation of the drug may result in deposits that can lead to blurred vision and blindness.[citation needed] It and related quinines have been associated with cases of retinal toxicity, particularly when provided at higher doses for longer times.[citation needed] With long-term doses, routine visits to an ophthalmologist are recommended.[citation needed]
Chloroquine is also a lysosomotropic agent, meaning it accumulates preferentially in the lysosomes of cells in the body.[citation needed] The pKa for the quinoline nitrogen of chloroquine is 8.5, meaning it is about 10% deprotonated at physiological pH (per the Henderson-Hasselbalch equation).[original research?][citation needed] This decreases to about 0.2% at a lysosomal pH of 4.6.[original research?][citation needed] Because the deprotonated form is more membrane-permeable than the protonated form, a quantitative "trapping" of the compound in lysosomes results.[citation needed]
Mechanism of action

Malaria
The lysosomotropic character of chloroquine is believed to account for much of its antimalarial activity; the drug concentrates in the acidic food vacuole of the parasite and interferes with essential processes. Its lysosomotropic properties further allow for its use for in vitro experiments pertaining to intracellular lipid related diseases,[26][27] autophagy, and apoptosis.[28]
Inside red blood cells, the malarial parasite, which is then in its asexual lifecycle stage, must degrade hemoglobin to acquire essential amino acids, which the parasite requires to construct its own protein and for energy metabolism. Digestion is carried out in a vacuole of the parasitic cell.[citation needed]
Hemoglobin is composed of a protein unit (digested by the parasite) and a heme unit (not used by the parasite). During this process, the parasite releases the toxic and soluble molecule heme. The heme moiety consists of a porphyrin ring called Fe(II)-protoporphyrin IX (FP). To avoid destruction by this molecule, the parasite biocrystallizes heme to form hemozoin, a nontoxic molecule. Hemozoin collects in the digestive vacuole as insoluble crystals.[citation needed]
Chloroquine enters the red blood cell by simple diffusion, inhibiting the parasite cell and digestive vacuole. Chloroquine then becomes protonated (to CQ2+), as the digestive vacuole is known to be acidic (pH 4.7); chloroquine then cannot leave by diffusion. Chloroquine caps hemozoin molecules to prevent further biocrystallization of heme, thus leading to heme buildup. Chloroquine binds to heme (or FP) to form the FP-chloroquine complex; this complex is highly toxic to the cell and disrupts membrane function. Action of the toxic FP-chloroquine and FP results in cell lysis and ultimately parasite cell autodigestion.[29] Parasites that do not form hemozoin are therefore resistant to chloroquine.[30]
Resistance in malaria
Since the first documentation of P. falciparum chloroquine resistance in the 1950s, resistant strains have appeared throughout East and West Africa, Southeast Asia, and South America. The effectiveness of chloroquine against P. falciparum has declined as resistant strains of the parasite evolved. They effectively neutralize the drug via a mechanism that drains chloroquine away from the digestive vacuole. Chloroquine-resistant cells efflux chloroquine at 40 times the rate of chloroquine-sensitive cells; the related mutations trace back to transmembrane proteins of the digestive vacuole, including sets of critical mutations in the P. falciparum chloroquine resistance transporter (PfCRT) gene. The mutated protein, but not the wild-type transporter, transports chloroquine when expressed in Xenopus oocytes (frog's eggs) and is thought to mediate chloroquine leak from its site of action in the digestive vacuole.[31] Resistant parasites also frequently have mutated products of the ABC transporter P. falciparum multidrug resistance (PfMDR1) gene, although these mutations are thought to be of secondary importance compared to Pfcrt. Verapamil, a Ca2+ channel blocker, has been found to restore both the chloroquine concentration ability and sensitivity to this drug. Recently, an altered chloroquine-transporter protein CG2 of the parasite has been related to chloroquine resistance, but other mechanisms of resistance also appear to be involved.[32] Research on the mechanism of chloroquine and how the parasite has acquired chloroquine resistance is still ongoing, as other mechanisms of resistance are likely.[citation needed]
Other agents which have been shown to reverse chloroquine resistance in malaria are chlorpheniramine, gefitinib, imatinib, tariquidar and zosuquidar.[33]
Antiviral
Chloroquine has antiviral effects.[34] It increases late endosomal and lysosomal pH, resulting in impaired release of the virus from the endosome or lysosome – release of the virus requires a low pH. The virus is therefore unable to release its genetic material into the cell and replicate.[35][36]
Chloroquine also seems to act as a zinc ionophore, that allows extracellular zinc to enter the cell and inhibit viral RNA-dependent RNA polymerase.[37][38]
Other
Chloroquine inhibits thiamine uptake.[39] It acts specifically on the transporter SLC19A3.
Against rheumatoid arthritis, it operates by inhibiting lymphocyte proliferation, phospholipase A2, antigen presentation in dendritic cells, release of enzymes from lysosomes, release of reactive oxygen species from macrophages, and production of IL-1.
History
In Peru, the indigenous people extracted the bark of the Cinchona tree (Cinchona officinalis)[40] and used the extract to fight chills and fever in the seventeenth century. In 1633 this herbal medicine was introduced in Europe, where it was given the same use and also began to be used against malaria.[41] The quinoline antimalarial drug quinine was isolated from the extract in 1820, and chloroquine is an analogue of this.
Chloroquine was discovered in 1934, by Hans Andersag and coworkers at the Bayer laboratories, who named it Resochin.[42] It was ignored for a decade, because it was considered too toxic for human use. Instead, the DAK used the chloroquine analogue 3-methyl-chloroquine, known as Sontochin. After Allied forces arrived in Tunis, Sontochin fell into the hands of Americans, who sent the material back to the United States for analysis, leading to renewed interest in chloroquine.[43][44] United States government-sponsored clinical trials for antimalarial drug development showed unequivocally that chloroquine has a significant therapeutic value as an antimalarial drug. It was introduced into clinical practice in 1947 for the prophylactic treatment of malaria.[45]
Society and culture

Formulations
Chloroquine comes in tablet form as the phosphate, sulfate, and hydrochloride salts. Chloroquine is usually dispensed as the phosphate.[46]
Names
Brand names include Chloroquine FNA, Resochin, Dawaquin, and Lariago.[47]
Other animals
Chloroquine, in various chemical forms, is used to treat and control surface growth of anemones and algae, and many protozoan infections in aquariums,[48] e.g. the fish parasite Amyloodinium ocellatum.[49]
Research
COVID-19
In late January 2020 during the 2019–20 coronavirus pandemic, Chinese medical researchers stated that exploratory research into chloroquine seemed to have "fairly good inhibitory effects" on the SARS-CoV-2 virus.[50] Requests to start clinical testing were submitted.[51] Use; however, is only recommended in the setting of an approved trial or under the details outlined by Monitored Emergency Use of Unregistered Interventions.[3]
Chloroquine has been recommended by Chinese, South Korean and Italian health authorities for the experimental treatment of COVID-19.[52][53] These agencies noted contraindications for people with heart disease or diabetes.[54]
On 24 March 2020, it was reported that one fatality and one hospitalization, a husband and wife, due to consumption of a fish tank antiparasitic containing chloroquine phosphate with the intention of prophylaxis against COVID-19.[55] Health experts warned against the misuse of the non-pharmaceutical versions of chloroquine phosphate since chloroquine has a relatively narrow therapeutic index and it can be toxic at levels not much higher than those used for treatment—which raises the risk of inadvertent overdose.[56][57]
On 27 March 2020, the FDA issued guidance, "do not use chloroquine phosphate intended for fish as treatment for COVID-19 in humans".[58]
On 1 April 2020, the European Medicines Agency (EMA) issued guidance that chloroquine and hydroxychloroquine are only to be used in clinical trials or emergency use programs.[59].
On the same date, initial results from a randomised controlled trial conducted in China that compared Chloroquine to Lopinavir/ritonavir were published. The study found evidence that Chloroquine was slightly more effective than Lopinavir/ritonavir at treating COVID-19 patients who met the study's enrollment criteria, and noted that patients treated with Chloroquine recovered better and regained their pulmonary function more quickly than patients treated with Lopinavir/ritonavir.[60]
Other viruses
Chloroquine had been also proposed as a treatment for SARS, with in vitro tests inhibiting the SARS-CoV virus.[61][62] In October 2004, a group of researchers at the Rega Institute for Medical Research published a report on chloroquine, stating that chloroquine acts as an effective inhibitor of the replication of the severe acute respiratory syndrome coronavirus (SARS-CoV) in vitro.[61]
Chloroquine was being considered in 2003, in pre-clinical models as a potential agent against chikungunya fever.[63]
Other
The radiosensitizing and chemosensitizing properties of chloroquine are beginning to be exploited in anticancer strategies in humans.[64][65] In biomedicinal science, chloroquine is used for in vitro experiments to inhibit lysosomal degradation of protein products.
References
- ^ a b c d e f g h i j k l m "Aralen Phosphate". The American Society of Health-System Pharmacists. Archived from the original on 8 December 2015. Retrieved 2 December 2015.
- ^ "Chloroquine Use During Pregnancy". Drugs.com. Archived from the original on 16 April 2019. Retrieved 16 April 2019.
There are no controlled data in human pregnancies.
- ^ a b Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (March 2020). "A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19". Journal of Critical Care. doi:10.1016/j.jcrc.2020.03.005. PMID 32173110.
- ^ Mittra, Robert A.; Mieler, William F. (2013). "Chapter 89 – Drug Toxicity of the Posterior Segment". Retina (Fifth ed.). W.B. Saunders. pp. 1532–1554. doi:10.1016/B978-1-4557-0737-9.00089-8. ISBN 978-1-4557-0737-9. Retrieved 25 March 2020.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Manson P, Cooke G, Zumla A, eds. (2009). Manson's tropical diseases (22nd ed.). [Edinburgh]: Saunders. p. 1240. ISBN 978-1-4160-4470-3. Archived from the original on 2 November 2018. Retrieved 9 September 2017.
- ^ Bhattacharjee, Mrinal (2016). Chemistry of Antibiotics and Related Drugs. Springer. p. 184. ISBN 978-3-319-40746-3. Archived from the original on 1 November 2018. Retrieved 9 September 2017.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06.
- ^ "Chloroquine (Base)". International Drug Price Indicator Guide. Archived from the original on 27 August 2018. Retrieved 4 December 2015.
- ^ "Frequently Asked Questions (FAQs): If I get malaria, will I have it for the rest of my life?". US Centers for Disease Control and Prevention. 8 February 2010. Archived from the original on 13 May 2012. Retrieved 14 May 2012.
- ^ Plowe CV (2005). "Antimalarial drug resistance in Africa: strategies for monitoring and deterrence". Malaria: Drugs, Disease and Post-genomic Biology. Current Topics in Microbiology and Immunology. Vol. 295. pp. 55–79. doi:10.1007/3-540-29088-5_3. ISBN 3-540-25363-7. PMID 16265887.
- ^ Uhlemann AC, Krishna S (2005). "Antimalarial multi-drug resistance in Asia: mechanisms and assessment". Malaria: Drugs, Disease and Post-genomic Biology. Current Topics in Microbiology and Immunology. Vol. 295. pp. 39–53. doi:10.1007/3-540-29088-5_2. ISBN 3-540-25363-7. PMID 16265886.
- ^ "Chloroquine phosphate tablet – chloroquine phosphate tablet, coated". dailymed.nlm.nih.gov. Archived from the original on 8 December 2015. Retrieved 4 November 2015.
- ^ CDC. Health information for international travel 2001–2002. Atlanta, Georgia: U.S. Department of Health and Human Services, Public Health Service, 2001.
- ^ Amebic Hepatic Abscesses~treatment at eMedicine
- ^ a b c d e f "Drugs & Medications". www.webmd.com. Retrieved 22 March 2020.
- ^ a b c d "Chloroquine Side Effects: Common, Severe, Long Term". Drugs.com. Retrieved 22 March 2020.
- ^ "Chloroquine: MedlinePlus Drug Information". medlineplus.gov. Retrieved 22 March 2020.
- ^ Ajayi AA (September 2000). "Mechanisms of chloroquine-induced pruritus". Clinical Pharmacology and Therapeutics. 68 (3): 336. PMID 11014416.
- ^ Vaziri A, Warburton B (1994). "Slow release of chloroquine phosphate from multiple taste-masked W/O/W multiple emulsions". Journal of Microencapsulation. 11 (6): 641–8. doi:10.3109/02652049409051114. PMID 7884629.
- ^ Tönnesmann E, Kandolf R, Lewalter T (June 2013). "Chloroquine cardiomyopathy – a review of the literature". Immunopharmacology and Immunotoxicology. 35 (3): 434–42. doi:10.3109/08923973.2013.780078. PMID 23635029.
- ^ a b c d e f g h i "Aralen Chloroquine Phosphate, USP" (PDF). Archived (PDF) from the original on 25 March 2020. Retrieved 24 March 2020.
- ^ a b "Malaria – Chapter 3 – 2016 Yellow Book". wwwnc.cdc.gov. Archived from the original on 14 January 2016. Retrieved 11 November 2015.
- ^ Ullberg S, Lindquist NG, Sjòstrand SE (September 1970). "Accumulation of chorio-retinotoxic drugs in the foetal eye". Nature. 227 (5264): 1257–8. Bibcode:1970Natur.227.1257U. doi:10.1038/2271257a0. PMID 5452818.
- ^ Cann HM, Verhulst HL (January 1961). "Fatal acute chloroquine poisoning in children". Pediatrics. 27: 95–102. PMID 13690445.
- ^ a b Molina DK (March 2012). "Postmortem hydroxychloroquine concentrations in nontoxic cases". The American Journal of Forensic Medicine and Pathology. 33 (1): 41–2. doi:10.1097/PAF.0b013e3182186f99. PMID 21464694.
- ^ Chen PM, Gombart ZJ, Chen JW (March 2011). "Chloroquine treatment of ARPE-19 cells leads to lysosome dilation and intracellular lipid accumulation: possible implications of lysosomal dysfunction in macular degeneration". Cell & Bioscience. 1 (1): 10. doi:10.1186/2045-3701-1-10. PMC 3125200. PMID 21711726.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, et al. (April 2010). "Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61". The Journal of Neuroscience. 30 (17): 5948–57. doi:10.1523/JNEUROSCI.0157-10.2010. PMC 2868326. PMID 20427654.
- ^ Kim EL, Wüstenberg R, Rübsam A, Schmitz-Salue C, Warnecke G, Bücker EM, et al. (April 2010). "Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells". Neuro-Oncology. 12 (4): 389–400. doi:10.1093/neuonc/nop046. PMC 2940600. PMID 20308316.
- ^ Hempelmann E (March 2007). "Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors". Parasitology Research. 100 (4): 671–6. doi:10.1007/s00436-006-0313-x. PMID 17111179.
- ^ Lin JW, Spaccapelo R, Schwarzer E, Sajid M, Annoura T, Deroost K, et al. (June 2015). "Replication of Plasmodium in reticulocytes can occur without hemozoin formation, resulting in chloroquine resistance" (PDF). The Journal of Experimental Medicine. 212 (6): 893–903. doi:10.1084/jem.20141731. PMC 4451122. PMID 25941254. Archived (PDF) from the original on 22 September 2017. Retrieved 4 November 2018.
- ^ Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K (September 2009). "Chloroquine transport via the malaria parasite's chloroquine resistance transporter". Science. 325 (5948): 1680–2. Bibcode:2009Sci...325.1680M. doi:10.1126/science.1175667. PMID 19779197.
- ^ Tripathi KD (2003). Essentials of Medical Pharmacology (fifth ed.). Jaypee Brothers Medical Publisher Ltd. pp. 739–740.
- ^ Alcantara LM, Kim J, Moraes CB, Franco CH, Franzoi KD, Lee S, et al. (June 2013). "Chemosensitization potential of P-glycoprotein inhibitors in malaria parasites". Experimental Parasitology. 134 (2): 235–43. doi:10.1016/j.exppara.2013.03.022. PMID 23541983.
- ^ Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (November 2003). "Effects of chloroquine on viral infections: an old drug against today's diseases?". The Lancet. Infectious Diseases. 3 (11): 722–7. doi:10.1016/s1473-3099(03)00806-5. PMID 14592603.
- ^ Al-Bari MA (February 2017). "Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases". Pharmacology Research & Perspectives. 5 (1): e00293. doi:10.1002/prp2.293. PMC 5461643. PMID 28596841.
- ^ Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV (November 2002). "Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus". Journal of Virology. 76 (22): 11440–6. doi:10.1128/JVI.76.22.11440-11446.2002. PMC 136743. PMID 12388705.
- ^ Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ (1 October 2014). "Chloroquine is a zinc ionophore". PloS One. 9 (10): e109180. doi:10.1371/journal.pone.0109180. PMC 4182877. PMID 25271834.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (November 2010). "Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture". PLoS Pathogens. 6 (11): e1001176. doi:10.1371/journal.ppat.1001176. PMC 2973827. PMID 21079686.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Huang Z, Srinivasan S, Zhang J, Chen K, Li Y, Li W, et al. (2012). "Discovering thiamine transporters as targets of chloroquine using a novel functional genomics strategy". PLOS Genetics. 8 (11): e1003083. doi:10.1371/journal.pgen.1003083. PMC 3510038. PMID 23209439.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Fern, Ken (2010–2020). "Cinchona officinalis – L." Plans for a Future. Archived from the original on 25 August 2017. Retrieved 2 February 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: date format (link) - ^ Kouznetsov, Vladímir (2008). "Antimalarials: construction of molecular hybrids based on chloroquine" (PDF). Universitas Scientiarum: 1. Archived (PDF) from the original on 22 February 2020. Retrieved 22 February 2020 – via scielo.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Krafts K, Hempelmann E, Skórska-Stania A (July 2012). "From methylene blue to chloroquine: a brief review of the development of an antimalarial therapy". Parasitology Research. 111 (1): 1–6. doi:10.1007/s00436-012-2886-x. PMID 22411634.
- ^ Sneader W (2005). Drug Discovery. A History. Wiley. ISBN 0-471-89980-1.
- ^ Pou S, Winter RW, Nilsen A, Kelly JX, Li Y, Doggett JS, et al. (July 2012). "Sontochin as a guide to the development of drugs against chloroquine-resistant malaria". Antimicrobial Agents and Chemotherapy. 56 (7): 3475–80. doi:10.1128/AAC.00100-12. PMC 3393441. PMID 22508305.
- ^ "The History of Malaria, an Ancient Disease". Centers for Disease Control. 29 July 2019. Archived from the original on 28 August 2010.
- ^ "Chloroquine". nih.gov. National Institutes of Health. Retrieved 24 March 2020.
- ^ "Ipca Laboratories: Formulations – Branded". Archived from the original on 6 April 2019. Retrieved 14 March 2020.
- ^ Hemdal, Jay. "Aquarium Fish: Chloroquine: A "New" Drug for Treating Fish Diseases". Advanced Aquarist. Archived from the original on 15 March 2013. Retrieved 26 March 2020.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Francis-Floyd, Ruth; Floyd, Maxine R. "Amyloodinium ocellatum, an Important Parasite of Cultured Marine Fish" (PDF). agrilife.org.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (March 2020). "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro". Cell Research. 30 (3): 269–271. doi:10.1038/s41422-020-0282-0. PMC 7054408. PMID 32020029.
- ^ "Could an old malaria drug help fight the new coronavirus?". asbmb.org. Archived from the original on 6 February 2020. Retrieved 6 February 2020.
- ^ "Physicians work out treatment guidelines for coronavirus". m.koreabiomed.com (in Korean). 13 February 2020. Archived from the original on 17 March 2020. Retrieved 18 March 2020.
- ^ "Azioni intraprese per favorire la ricerca e l'accesso ai nuovi farmaci per il trattamento del COVID-19". aifa.gov.it (in Italian). Retrieved 18 March 2020.
- ^ "Plaquenil (hydroxychloroquine sulfate) dose, indications, adverse effects, interactions... from PDR.net". www.pdr.net. Archived from the original on 18 March 2020. Retrieved 19 March 2020.
- ^ "A man died after ingesting a substance he thought would protect him from coronavirus". NBC News. Retrieved 25 March 2020.
- ^ "Severe Illness Associated with Using Non-Pharmaceutical Chloroquine Phosphate to Prevent and Treat Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 28 March 2020. Retrieved 31 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Banner Health experts warn against self-medicating to prevent or treat COVID-19". Banner Health (Press release). 23 March 2020. Retrieved 25 March 2020.
- ^ "FDA Letter to Stakeholders: Do Not Use Chloroquine Phosphate Intended for Fish as Treatment for COVID-19 in Humans". U.S. Food and Drug Administration (FDA). 27 March 2020. Retrieved 1 April 2020.
- ^ "COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes". European Medicines Agency (EMA). 1 April 2020. Retrieved 2 April 2020.
- ^ Huang, Mingxing; Tang, Tiantian; Pang, Pengfei; Li, Man; Ma, Ruolan; Lu, Jiahui; Shu, Jingxian; You, Yingying; Chen, Binghui (1 April 2020). "Treating COVID-19 with Chloroquine". Journal of Molecular Cell Biology. doi:10.1093/jmcb/mjaa014. PMID 32236562. Retrieved 3 April 2020.
- ^ a b Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M (October 2004). "In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine". Biochemical and Biophysical Research Communications. 323 (1): 264–8. doi:10.1016/j.bbrc.2004.08.085. PMID 15351731.
- ^ Devaux CA, Rolain JM, Colson P, Raoult D (March 2020). "New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?". International Journal of Antimicrobial Agents: 105938. doi:10.1016/j.ijantimicag.2020.105938. PMID 32171740.
- ^ Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R (November 2003). "Effects of chloroquine on viral infections: an old drug against today's diseases?". The Lancet. Infectious Diseases. 3 (11): 722–7. doi:10.1016/S1473-3099(03)00806-5. PMID 14592603.
- ^ Savarino A, Lucia MB, Giordano F, Cauda R (October 2006). "Risks and benefits of chloroquine use in anticancer strategies". The Lancet. Oncology. 7 (10): 792–3. doi:10.1016/S1470-2045(06)70875-0. PMID 17012039.
- ^ Sotelo J, Briceño E, López-González MA (March 2006). "Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial". Annals of Internal Medicine. 144 (5): 337–43. doi:10.7326/0003-4819-144-5-200603070-00008. PMID 16520474.
"Summaries for patients. Adding chloroquine to conventional chemotherapy and radiotherapy for glioblastoma multiforme". Annals of Internal Medicine. 144 (5): I31. March 2006. doi:10.7326/0003-4819-144-5-200603070-00004. PMID 16520470.
External links
- "Chloroquine". Drug Information Portal. U.S. National Library of Medicine.
- "Medicines for the Prevention of Malaria While Traveling – Chloroquine (Aralen)" (PDF) (Fact sheet). U.S. Centers for Disease Control and Prevention (CDC).
 The dictionary definition of chloroquine at Wiktionary
The dictionary definition of chloroquine at Wiktionary
