Technetium
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Technetium | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /tɛkˈniːʃ(i)əm/ | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | shiny gray metal | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass number | [97] (data not decisive)[a] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Technetium in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 43 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d5 5s2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 13, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2430 K (2157 °C, 3915 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4538 K (4265 °C, 7709 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 98Tc: 11.359 g/cm3 99Tc: 11.475 g/cm3[2] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 33.29 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 585.2 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.27 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure (extrapolated)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +4, +7 −3,[3] −1,[3] +1,[3] +2,[3] +3,[3] +5,[3] +6[3] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 136 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 147±7 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 205 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 274.12 pm c = 439.90 pm (at 20 °C)[2] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 8.175×10−6/K (at 20 °C)[2] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 50.6 W/(m⋅K) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 200 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | Paramagnetic | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +270.0×10−6 cm3/mol (298 K)[5] | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 16,200 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-26-8 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prediction | Dmitri Mendeleev (1871) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Emilio Segrè and Carlo Perrier (1937) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of technetium | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Technetium (/[invalid input: 'icon']tɛkˈniːʃiəm/ tek-NEE-shee-əm) is the chemical element with atomic number 43 and symbol Tc. It is the lowest atomic number element without any stable isotopes; every form of it is radioactive. Nearly all technetium is produced synthetically and only minute amounts are found in nature. Naturally occurring technetium occurs as a spontaneous fission product in uranium ore or by neutron capture in molybdenum ores. The chemical properties of this silvery gray, crystalline transition metal are intermediate between rhenium and manganese.
Many of technetium's properties were predicted by Dmitri Mendeleev before the element was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name ekamanganese (Em). In 1937 technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produced, hence its name (from the Greek [τεχνητός] Error: {{Lang}}: text has italic markup (help), meaning "artificial").
Its short-lived gamma ray-emitting nuclear isomer—technetium-99m—is used in nuclear medicine for a wide variety of diagnostic tests. Technetium-99 is used as a gamma ray-free source of beta particles. Long-lived technetium isotopes produced commercially are by-products of fission of uranium-235 in nuclear reactors and are extracted from nuclear fuel rods. Because no isotope of technetium has a half-life longer than 4.2 million years (technetium-98), its detection in red giants in 1952, which are billions of years old, helped bolster the theory that stars can produce heavier elements.
History
Search for element 43
From the 1860s through 1871, early forms of the periodic table proposed by Dimitri Mendeleev contained a gap between molybdenum (element 42) and ruthenium (element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below manganese and therefore be chemically similar to manganese. Mendeleev gave it the provisional name ekamanganese (eka- from the Sanskrit words for one), because the predicted element was one place down from the known element manganese.[6]
Many early researchers, both before and after the periodic table was published, were eager to be the first to discover and name the missing element; its location in the table suggested that it should be easier to find than other undiscovered elements. It was first thought to have been found in platinum ores in 1828 and was given the name polinium, but turned out to be impure iridium. Then, in 1846, the element ilmenium was claimed to have been discovered, but later was determined to be impure niobium. This mistake was repeated in 1847 with the "discovery" of pelopium.[7]
In 1877, the Russian chemist Serge Kern reported discovering the missing element in platinum ore. Kern named what he thought was the new element davyum (after the noted English chemist Sir Humphry Davy), but it was eventually determined to be a mixture of iridium, rhodium and iron. Another candidate, lucium, followed in 1896, but it was determined to be yttrium. Then in 1908, the Japanese chemist Masataka Ogawa found evidence in the mineral thorianite, which he thought indicated the presence of element 43. Ogawa named the element nipponium, after Japan (which is Nippon in Japanese). In 2004, H. K Yoshihara used "a record of X-ray spectrum of Ogawa's nipponium sample from thorianite [which] was contained in a photographic plate preserved by his family. The spectrum was read and indicated the absence of the element 43 and the presence of the element 75 (rhenium)."[8]
German chemists Walter Noddack, Otto Berg, and Ida Tacke reported the discovery of element 75 and element 43 in 1925, and named element 43 masurium (after Masuria in eastern Prussia, now in Poland, the region where Walter Noddack's family originated).[9] The group bombarded columbite with a beam of electrons and deduced element 43 was present by examining X-ray diffraction spectrograms.[10] The wavelength of the X-rays produced is related to the atomic number by a formula derived by Henry Moseley in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error for many years.[11][12] Still, in 1933, a series of articles on the discovery of elements quoted the name masurium for element 43.[13][note 1] Debate still exists as to whether the 1925 team actually did discover element 43.[14]
Official discovery and later history
The discovery of element 43 was finally confirmed in a December 1936 experiment at the University of Palermo in Sicily conducted by Carlo Perrier and Emilio Segrè.[15] In mid-1936, Segrè visited the United States, first Columbia University in New York and then the Lawrence Berkeley National Laboratory in California. He persuaded cyclotron inventor Ernest Lawrence to let him take back some discarded cyclotron parts that had become radioactive. Lawrence mailed him a molybdenum foil that had been part of the deflector in the cyclotron.
Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed Z = 43. They succeeded in isolating the isotopes technetium-95 and technetium-97.[16][17] University of Palermo officials wanted them to name their discovery "panormium", after the Latin name for Palermo, Panormus. In 1947[16] element 43 was named after the Greek word τεχνητός, meaning "artificial", since it was the first element to be artificially produced.[7][9] Segrè returned to Berkeley and met Glenn T. Seaborg. They isolated the metastable isotope technetium-99m, which is now used in some ten million medical diagnostic procedures annually.[18]
In 1952, astronomer Paul W. Merrill in California detected the spectral signature of technetium (in particular, light with wavelength of 403.1 nm, 423.8 nm, 426.8 nm, and 429.7 nm) in light from S-type red giants.[19] The stars were near the end of their lives, yet were rich in this short-lived element, meaning nuclear reactions within the stars must be producing it. This evidence was used to bolster the then-unproven theory that stars are where nucleosynthesis of the heavier elements occurs.[17] More recently, such observations provided evidence that elements were being formed by neutron capture in the s-process.[20]
Since its discovery, there have been many searches in terrestrial materials for natural sources of technetium. In 1962, technetium-99 was isolated and identified in pitchblende from the Belgian Congo in extremely small quantities (about 0.2 ng/kg);[20] there it originates as a spontaneous fission product of uranium-238. There is also evidence that the Oklo natural nuclear fission reactor produced significant amounts of technetium-99, which has since decayed into ruthenium-99.[20]
Characteristics
Physical properties
Technetium is a silvery-gray radioactive metal with an appearance similar to that of platinum. It is commonly obtained as a gray powder.[21] The crystal structure of the pure metal is hexagonal close-packed. Atomic technetium has characteristic emission lines at these wavelengths of light: 363.3 nm, 403.1 nm, 426.2 nm, 429.7 nm, and 485.3 nm.[22]
The metal form is slightly paramagnetic, meaning its magnetic dipoles align with external magnetic fields, but will assume random orientations once the field is removed.[23] Pure, metallic, single-crystal technetium becomes a type-II superconductor at temperatures below 7.46 K.[note 2][24] Below this temperature, technetium has a very high magnetic penetration depth, the largest among the elements apart from niobium.[25]
Chemical properties
Technetium is placed in the seventh group of the periodic table, between rhenium and manganese. As predicted by periodic law, its chemical properties are therefore intermediate between those two elements. Of the two, technetium more closely resembles rhenium, particularly in its chemical inertness and tendency to form covalent bonds.[26] Unlike manganese, technetium does not readily form cations (ions with a net positive charge). Common oxidation states of technetium include +4, +5, and +7.[27] Technetium dissolves in aqua regia, nitric acid, and concentrated sulfuric acid, but it is not soluble in hydrochloric acid of any concentration.[21]
Hydride and oxides
Reaction of technetium with hydrogen produces the negatively charged hydride [TcH9]2− ion, which has the same type of crystal structure as (isostructural with) [ReH9]2−. It consists of a trigonal prism with a technetium atom in the center and six hydrogen atoms at the corners. Three more hydrogens make a triangle lying parallel to the base and crossing the prism in its center. Although those hydrogen atoms are not equivalent geometrically, their electronic structure is almost the same. This complex has a coordination number of 9 (meaning that the Tc atom has nine neighbors), which is the highest for a technetium complex. Two hydrogen atoms in the complex can be replaced by sodium (Na+) or potassium (K+) ions.[28]
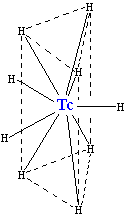
The metal form of technetium slowly tarnishes in moist air,[27] and in powder form will burn in oxygen. Two oxides have been observed: TcO2 and Tc2O7. Under oxidizing conditions, which tend to strip electrons from atoms, technetium(VII) will exist as the pertechnetate ion, TcO−
4.[23][27]
At temperatures of 400–450 °C, technetium oxidizes to form pale-yellow heptoxide:
- 4 Tc + 7 O2 → 2 Tc2O7
It adopts a centrosymmetric structure with two types of Tc-O bonds; their bond lengths are 167 and 184 pm, and the O-Tc-O angle is 180°.[29]
Technetium heptoxide is the precursor to sodium pertechnetate:[30]
- Tc2O7 + 2 NaOH → 2 NaTcO4 + H2O
Black-colored technetium dioxide (TcO2) can be produced by reduction of heptoxide with technetium or hydrogen.[31]
Pertechnetic acid (HTcO4) is produced by reacting Tc2O7 with water or oxidizing acids, such as nitric acid, concentrated sulfuric acid, aqua regia, or a mixture of nitric and hydrochloric acids.[32] The resulting dark red, hygroscopic (water absorbing) substance is a strong acid and easily donates protons.
The remaining pertechnate anion TcO4− consists of a tetrahedron with oxygens in the corners and Tc atom in the center. Unlike permanganate (MnO4−), it is only a weak oxidizing agent. Pertechnate is often used as a convenient water-soluble source of Tc isotopes, such as 99mTc, and as a catalyst.[33]
Sulfides, selenides, tellurides
Technetium forms various sulfides. TcS2 is obtained by direct reaction of technetium and elemental sulfur, while Tc2S7 is formed from the pertechnic acid as follows:
- 2 HTcO4 + 7 H2S → Tc2S7 + 8 H2O
In this reaction, technetium is not reduced, which is different from the similar reaction of manganese. Upon heating, technetium heptasulfide decomposes into disulfide and elementary sulfur:
- Tc2S7 → 2 TcS2 + 3 S
Analogous reactions occur with selenium and tellurium.[34]

Clusters and organic complexes
Several technetium clusters are known, including Tc4, Tc6, Tc8 and Tc13.[35][36] The more stable Tc6 and Tc8 clusters have prism shapes where vertical pairs of Tc atoms are connected by triple bonds and the planar atoms by single bonds. Every Tc atom makes six bonds, and the remaining valence electrons can be saturated by one axial and two bridging ligand halogen atoms such as chlorine or bromine.[37]

Technetium forms numerous organic complexes, which are relatively well-investigated because of their importance for nuclear medicine. Technetium carbonyl (Tc2(CO)10) is a white solid.[39] In this molecule, two technetium atoms are weakly bound to each other; each atom is surrounded by octahedra of five carbonyl ligands. The bond length between Tc atoms, 303 pm,[40][41] is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar carbonyls are formed by manganese and rhenium.[42]
A technetium complex[note 3] with an organic ligand (shown in the figure on right) is commonly used in nuclear medicine. It has a unique Tc-O functional group (moiety) oriented perpendicularly to the plane of the molecule, where the oxygen atom can be replaced by a nitrogen atom.[43]
Isotopes
Technetium, atomic number (Z) 43, is the lowest-numbered element in the periodic table that is exclusively radioactive. The second-lightest radioactive element, promethium, has an atomic number of 61.[27] Atomic nuclei with an odd number of protons are less stable than those with even numbers, even when the total number of nucleons (protons + neutrons) are even.[44] Odd numbered elements therefore have fewer stable isotopes.
The most stable radioactive isotopes are technetium-98 with a half-life of 4.2 million years (Ma), technetium-97 (half-life: 2.6 Ma) and technetium-99 (half-life: 211,000 years).[45] Thirty other radioisotopes have been characterized with mass numbers ranging from 85 to 118.[45] Most of these have half-lives that are less than an hour; the exceptions are technetium-93 (half-life: 2.73 hours), technetium-94 (half-life: 4.88 hours), technetium-95 (half-life: 20 hours), and technetium-96 (half-life: 4.3 days).[46]
The primary decay mode for isotopes lighter than technetium-98 (98
43Tc
) is electron capture, giving molybdenum (Z=42).[45] For heavier isotopes, the primary mode is beta emission (the loss of an electron or positron), giving ruthenium (Z=44), with the exception that technetium-100 can decay both by beta emission and electron capture.[45][47]
Technetium also has numerous nuclear isomers, which are isotopes with one or more excited nucleons. Technetium-97m (97mTc ; 'm' stands for metastability) is the most stable, with a half-life of 91 days (0.0965 MeV).[46] This is followed by technetium-95m (half life: 61 days, 0.03 MeV), and technetium-99m (half-life: 6.01 hours, 0.142 MeV).[46] Technetium-99m only emits gamma rays and decays to technetium-99.[46]
Technetium-99 (99
43Tc
) is a major product of the fission of uranium-235 (235
92U
), making it the most common and most readily available Tc isotope. One gram of technetium-99 produces 6.2×108 disintegrations a second (that is, 0.62 GBq/g).[23]
Occurrence and production

Since technetium is unstable, only minute traces occur naturally in the Earth's crust as a spontaneous fission product in uranium ores. A kilogram of uranium contains an estimated 1 nanogram (10−9 g) of technetium.[17][48][49] Some red giant stars with the spectral types S-, M-, and N contain an absorption line in their spectrum indicating the presence of technetium.[21][50] These red-giants are known informally as technetium stars.
Fission waste product
In contrast with its rare natural occurrence, bulk quantities of technetium-99 are produced each year from spent nuclear fuel rods, which contain various fission products. The fission of a gram of uranium-235 in nuclear reactors yields 27 mg of technetium-99, giving technetium a fission product yield of 6.1%.[23] Other fissile isotopes also produce similar yields of technetium, such as 4.9% from uranium-233 and 6.21% from plutonium-239.[51] About 49,000 TBq (78 metric tons) of technetium is estimated to have been produced in nuclear reactors between 1983 and 1994, which is by far the dominant source of terrestrial technetium.[52][53] Only a fraction of the production is used commercially.[note 4]
Technetium-99 is produced by the nuclear fission of both uranium-235 and plutonium-239. It is therefore present in radioactive waste and in the nuclear fallout of fission bomb explosions. Its decay, measured in becquerels per amount of spent fuel, is dominant after about 104 to 106 years after the creation of the nuclear waste.[52] From 1945 to 1994, an estimated 160 TBq (about 250 kg) of technetium-99 was released into the environment by atmospheric nuclear tests.[52][54] The amount of technetium-99 from nuclear reactors released into the environment up to 1986 is on the order of 1000 TBq (about 1600 kg), primarily by nuclear fuel reprocessing; most of this was discharged into the sea. Reprocessing methods have reduced emissions since then, but as of 2005 the primary release of technetium-99 into the environment is by the Sellafield plant, which released an estimated 550 TBq (about 900 kg) from 1995–1999 into the Irish Sea.[53] From 2000 onwards the amount has been limited by regulation to 90 TBq (about 140 kg) per year.[55] Discharge of technetium into the sea has resulted in some seafood containing minuscule quantities of this element. For example, European lobster and fish from west Cumbria contain about 1 Bq/kg of technetium.[56][57][note 5]
Fission product for commercial use
The vast majority of the technetium-99m used in medical work is produced by irradiating highly enriched uranium targets in a reactor, extracting molybdenum-99 from the targets, and recovering the technetium-99m that is produced upon decay of molybdenum-99.[58]
Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at Chalk River Laboratories reactor in Ontario, Canada, and the Petten nuclear reactor of the Netherlands. All major technetium-99m producing reactors were built in the 1960s and are close to the end of their lifetime. The two new Canadian Multipurpose Applied Physics Lattice Experiment reactors planned and built to produce 200% of the demand of technetium-99m relieved all other producers from building their own reactors. With the cancellation of the already tested reactors in 2008 the future supply of technetium-99m became very problematic.[59]
However the Chalk River reactor has been shut down for maintenance since August 2009, with an expected reopening in April 2010, and the Petter reactor has a 6-month scheduled maintenance shutdown beginning on Friday, February 19, 2010. With millions of procedures relying on technetium-99m every year, the low supply has left a gap, leaving some practitioners to revert to techniques not used for 20 years. Somewhat allaying this issue is an announcement from a Polish research reactor, the Maria, that they have developed a technique to isolate technetium.[60] The reactor at Chalk River Laboratory reopened in August 2010 and the Petten reactor reopened September 2010.[61]
Waste disposal
The long half-life of technetium-99 and its ability to form an anionic species makes it a major concern for long-term disposal of radioactive waste. Many of the processes designed to remove fission products in reprocessing plants aim at cationic species like caesium (e.g., caesium-137) and strontium (e.g., strontium-90). Hence the pertechnetate is able to escape through these treatment processes. Current disposal options favor burial in continental, geologically stable rock. The primary danger with such a course is that the waste is likely to come into contact with water, which could leach radioactive contamination into the environment. The anionic pertechnetate and iodide do not adsorb well onto the surfaces of minerals, so they are likely to be washed away. By comparison plutonium, uranium, and caesium are much more able to bind to soil particles. For this reason, the environmental chemistry of technetium is an active area of research.[62]
An alternative disposal method, transmutation, has been demonstrated at CERN for technetium-99. This transmutation process is one in which the technetium (technetium-99 as a metal target) is bombarded with neutrons to form the short-lived technetium-100 (half life = 16 seconds) which decays by beta decay to ruthenium-100. If recovery of usable ruthenium is a goal, an extremely pure technetium target is needed; if small traces of the minor actinides such as americium and curium are present in the target, they are likely to undergo fission and form more fission products which increase the radioactivity of the irradiated target. The formation of ruthenium-106 (half-life 374 days) from the 'fresh fission' is likely to increase the activity of the final ruthenium metal, which will then require a longer cooling time after irradiation before the ruthenium can be used.[63]
The actual production of technetium-99 from spent nuclear fuel is a long process. During fuel reprocessing, it appears in the waste liquid, which is highly radioactive. After sitting for several years, the radioactivity falls to a point where extraction of the long-lived isotopes, including technetium-99, becomes feasible. Several chemical extraction processes are then used, yielding technetium-99 metal of high purity.[64]
Neutron activation
The metastable isotope technetium-99m is produced as a fission product from the fission of uranium or plutonium in nuclear reactors. Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m will have decayed by the time that the fission products are separated from the major actinides in conventional nuclear reprocessing. The liquid left after plutonium–uranium extraction (PUREX) contains a high concentration of technetium as TcO−
4 but almost all of this is technetium-99, not technetium-99m.[65]
Molybdenum-99 can be formed by the neutron activation of molybdenum-98.[66] Molybdenum-99 has a half-life of 67 hours, so short-lived technetium-99m (half-life: 6 hours), which results from its decay, is being constantly produced.[17] The technetium can then be chemically extracted from the solution by using a technetium-99m generator ("technetium cow", also occasionally called a "molybdenum cow"). By irradiating a highly enriched uranium target to produce molybdenum-99, there is no need for the complex chemical steps which would be required to separate molybdenum from a fission product mixture. This method requires that an enriched uranium target be irradiated with neutron to form molybdenum-99 as a fission product, then separated.[67] A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.[68]
Other technetium isotopes are not produced in significant quantities by fission; when needed, they are manufactured by neutron irradiation of parent isotopes (for example, technetium-97 can be made by neutron irradiation of ruthenium-96).[69]
Applications
Nuclear medicine and biology
Technetium-99m ("m" indicates that this is a metastable nuclear isomer) is used in radioactive isotope medical tests, for example as a radioactive tracer that medical equipment can detect in the human body.[17] It is well suited to the role because it emits readily detectable 140 keV gamma rays, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours).[23] There are at least 31 commonly used radiopharmaceuticals based on technetium-99m for imaging and functional studies of the brain, myocardium, thyroid, lungs, liver, gallbladder, kidneys, skeleton, blood, and tumors.[70]
The longer-lived isotope technetium-95m, with a half-life of 61 days, is used as a radioactive tracer to study the movement of technetium in the environment and in plant and animal systems.[71]
Industrial and chemical
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma rays. Moreover, its long half-life means that this emission decreases very slowly with time. It can also be extracted to a high chemical and isotopic purity from radioactive waste. For these reasons, it is a National Institute of Standards and Technology (NIST) standard beta emitter, and is therefore used for equipment calibration.[72] Technetium-99 has also been proposed for use in optoelectronic devices and nanoscale nuclear batteries.[73]
Like rhenium and palladium, technetium can serve as a catalyst. For some reactions, for example the dehydrogenation of isopropyl alcohol, it is a far more effective catalyst than either rhenium or palladium. However, its radioactivity is a major problem in finding safe catalytic applications.[74]
When steel is immersed in water, adding a small concentration (55 ppm) of potassium pertechnetate(VII) to the water protects the steel from corrosion, even if the temperature is raised to 250 °C.[75] For this reason, pertechnetate has been used as a possible anodic corrosion inhibitor for steel, although technetium's radioactivity poses problems which limit this application to self-contained systems.[76] While (for example) CrO42− can also inhibit corrosion, it requires a concentration ten times as high. In one experiment, a specimen of carbon steel was kept in an aqueous solution of pertechnetate for 20 years and was still uncorroded.[75] The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer. One theory holds that the pertechnetate reacts with the steel surface to form a layer of technetium dioxide which prevents further corrosion; the same effect explains how iron powder can be used to remove pertechnetate from water. (Activated carbon can also be used for the same effect.) The effect disappears rapidly if the concentration of pertechnetate falls below the minimum concentration or if too high a concentration of other ions is added.[77]
As noted, the radioactive nature of technetium (3 MBq per liter at the concentrations required) makes this corrosion protection impractical in almost all situations. Nevertheless, corrosion protection by pertechnetate ions was proposed (but never adopted) for use in boiling water reactors.[77]
Precautions
Technetium plays no natural biological role and is not normally found in the human body.[21] Technetium is produced in quantity by nuclear fission, and spreads more readily than many radionuclides. It appears to have low chemical toxicity. For example, no significant change in blood formula, body and organ weights, and food consumption could be detected for rats which ingested up to 15 µg of technetium-99 per gram of food for several weeks.[78] The radiological toxicity of technetium (per unit of mass) is a function of compound, type of radiation for the isotope in question, and the isotope's half-life.[79]
All isotopes of technetium must be handled carefully. The most common isotope, technetium-99, is a weak beta emitter; such radiation is stopped by the walls of laboratory glassware. The primary hazard when working with technetium is inhalation of dust; such radioactive contamination in the lungs can pose a significant cancer risk. For most work, careful handling in a fume hood is sufficient; a glove box is not needed.[80]
Notes
- ^ In 1998 John T. Armstrong of the National Institute of Standards and Technology ran "computer simulations" of the 1925 experiments and obtained results quite close to those reported by the Noddack team. "Using first-principles X-ray-emission spectral-generation algorithms developed at NIST, I simulated the X-ray spectra that would be expected for Van Assche's initial estimates of the Noddacks' residue compositions. The first results were surprisingly close to their published spectrum! Over the next couple of years, we refined our reconstruction of their analytical methods and performed more sophisticated simulations. The agreement between simulated and reported spectra improved further. Our calculation of the amount of element 43 required to produce their spectrum is quite similar to the direct measurements of natural technetium abundance in uranium ore published in 1999 by Dave Curtis and colleagues at Los Alamos. We can find no other plausible explanation for the Noddacks' data than that they did indeed detect fission "masurium."
Armstrong, J. T. (2003). "Technetium". Chemical & Engineering News. - ^ Irregular crystals and trace impurities raise this transition temperature to 11.2 K for 99.9% pure technetium powder.(Schwochau 2000, p. 96)
- ^ 3,3,9,9-tetramethyl-4,8-diazaundecane-2,lO-dion dioxime Hexamethyipropyleneamine Oxime (HMPAO)
- ^ As of 2005[update], technetium-99 in the form of ammonium pertechnate is available to holders of an Oak Ridge National Laboratory permit:Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0849304857.
- ^ The anaerobic, spore-forming bacteria in the Clostridium genus are able to reduce Tc(VII) to Tc(IV). Clostridia bacteria play a role in reducing iron, manganese, and uranium, thereby affecting these elements' solubility in soil and sediments. Their ability to reduce technetium may determine a large part of mobility of technetium in industrial wastes and other subsurface environments. Francis, A. J.; Dodge, C. J.; Meinken, G. E. (2002). "Biotransformation of pertechnetate by Clostridia". Radiochimica Acta. 90: 791–797. doi:10.1524/ract.2002.90.9-11_2002.791.
References
- ^ a b Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d e f g Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ Mattolat, C.; Gottwald, T.; Raeder, S.; Rothe, S.; Schwellnus, F.; Wendt, K.; Thörle-Pospiech, P.; Trautmann, N. (24 May 2010). "Determination of the first ionization potential of technetium". Physical Review A. 81: 052513. doi:10.1103/PhysRevA.81.052513.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Jonge, F. A. A.; Pauwels, EK (1996). "Technetium, the missing element". European Journal of Nuclear Medicine. 23 (3): 336. doi:10.1007/BF00837634. PMID 8599967.
- ^ a b Holden, N. E. "History of the Origin of the Chemical Elements and Their Discoverers". Brookhaven National Laboratory. Retrieved 2009-05-05.
- ^ Yoshihara, H. K. (2004). "Discovery of a new element 'nipponium': re-evaluation of pioneering works of Masataka Ogawa and his son Eijiro Ogawa". Atomic spectroscopy (Spectrochim. Acta, Part B). 59 (8): 1305–1310. doi:10.1016/j.sab.2003.12.027.
- ^ a b van der Krogt, P. "Elentymolgy and Elements Multidict, "Technetium"". Retrieved 2009-05-05.
- ^ Emsley 2001, p. 423 harvnb error: multiple targets (2×): CITEREFEmsley2001 (help)
- ^ Armstrong, J. T. (2003). "Technetium". Chemical & Engineering News. Retrieved 2009-11-11.
- ^ Nies, K. A. (2001). "Ida Tacke and the warfare behind the discovery of fission". Retrieved 2009-05-05.
- ^ Weeks, M. E. (1933). "The discovery of the elements. XX. Recently discovered elements". Journal of Chemical Education: 161–170.
- ^ Zingales, R. (2005). "From Masurium to Trinacrium: The Troubled Story of Element 43". Journal of Chemical Education. 82: 221–227. doi:10.1021/ed082p221.
- ^ Heiserman 1992, p. 164
- ^ a b Perrier, C.; Segrè, E. (1947). "Technetium: The Element of Atomic Number 43". Nature. 159 (4027): 24. doi:10.1038/159024a0. PMID 20279068.
- ^ a b c d e Emsley, J. (2001). Nature's Building Blocks: An A-Z Guide to the Elements. New York: Oxford University Press. pp. 422–425. ISBN 0-19-850340-7.
- ^ "Chapter 1.2: Early Days at the Berkeley Radiation Laboratory". The transuranium people: The inside story. University of California, Berkeley & Lawrence Berkeley National Laboratory. 2000. p. 15. ISBN 1-86094-087-0.
- ^ Merrill, P. W. (1952). "Technetium in the stars". Science. 115: 484.
- ^ a b c Schwochau 2000, pp. 7–9
- ^ a b c d Hammond, C. R. (2004). "The Elements". Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 0849304857.
- ^ Lide, David R. (2004–2005). "Line Spectra of the Elements". The CRC Handbook. CRC press. pp. 10–70 (1672). ISBN 9780849305955.
- ^ a b c d e Rimshaw, S. J. (1968). Hampel, C. A. (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation. pp. 689–693.
- ^ Schwochau 2000, p. 96
- ^ Autler, S. H. "Technetium as a Material for AC Superconductivity Applications" (PDF). Proceedings of the 1968 Summer Study on Superconducting Devices and Accelerators. Retrieved 2009-05-05.
- ^ Greenwood 1997, p. 1044
- ^ a b c d Husted, R. (2003-12-15). "Technetium". Periodic Table of the Elements. Los Alamos National Laboratory. Retrieved 2009-10-11.
- ^ Schwochau 2000, p. 146
- ^ Krebs, B. (1969). "Technetium(VII)-oxid: Ein Übergangsmetalloxid mit Molekülstruktur im festen Zustand". Angewandte Chemie. 81: 328–329. doi:10.1002/ange.19690810905.
- ^ Herrell, A. Y.; Busey, R. H.; Gayer, K. H. (1977). Technetium(VII) Oxide, in Inorganic Syntheses. Vol. XVII. pp. 155–158. ISBN 0-07-044327-0.
- ^ Schwochau 2000, p. 108
- ^ Schwochau 2000, p. 127
- ^ Schwochau 2000, pp. 127–136
- ^ Schwochau 2000, pp. 112–113
- ^ Cotton 1999, p. 985
- ^ Löwdin, P.-O.; Brändas, E.; Kryachko, E. S. (2003). Fundamental world of quantum chemistry: a tribute to the memory of Per-Olov Löwdin. Vol. 2. Springer. p. 479. ISBN 1402012861.
- ^ German, K. E.; Kryutchkov, S. V. (2002). "Polynuclear Technetium Halide Clusters". Russian Journal of Inorganic Chemistry. 47 (4): 578–583.
- ^ 3,3,9,9-Tetramethyl-4,8-diazaundecane-2,10-dione dioximato-oxotechnetium(V) [TcO(pnao)]; Schwochau 2000, p. 176
- ^ Hileman, J. C.; Huggins, D. K.; Kaesz, H. D. (1961). "Technetium carbonyl". Journal of the American Chemical Society. 83: 2953–2954. doi:10.1021/ja01474a038.
- ^ Bailey, M. F.; Dahl, Lawrence F. (1965). "The Crystal Structure of Ditechnetium Decacarbonyl". Inorganic Chemistry. 4: 1140–1145. doi:10.1021/ic50030a011.
- ^ Wallach, D. (1962). "Unit cell and space group of technetium carbonyl, Tc2(CO)10". Acta Crystallographica. 15: 1058. doi:10.1107/S0365110X62002789.
- ^ Schwochau 2000, pp. 286, 328
- ^ Jurisson, S.; Schlemper, E. O.; Troutner, D. E.; Canning, L. R.; Nowotnik, D. P.; Neirinckx, R. D. (1986). "Synthesis, characterization, and x-ray structural determinations of technetium(V)-oxo-tetradentate amine oxime complexes". Inorganic Chemistry. 25: 543–549. doi:10.1021/ic00224a031.
- ^ Clayton, D. D. (1983). Principles of stellar evolution and nucleosynthesis: with a new preface. University of Chicago Press. p. 547. ISBN 0-226-10953-4.
- ^ a b c d NNDC contributors (2008). Sonzogni, A. A. (ed.). "Chart of Nuclides". New York: National Nuclear Data Center, Brookhaven National Laboratory. Retrieved 2009-11-11.
{{cite web}}:|author=has generic name (help) - ^ a b c d Holden, N. E. (2006). Lide. D. R. (ed.). Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press, Taylor & Francis Group. pp. 11–88–11–89. ISBN 0-8493-0487-3.
- ^ Lide, David R., ed. (2004–2005). "Table of the isotopes". The CRC Handbook of Chemistry and Physics. CRC press.
- ^ Dixon, P.; Curtis, David B.; Musgrave, John; Roensch, Fred; Roach, Jeff; Rokop, Don; et al. (1997). "Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials". Analytical Chemistry. 69: 1692. doi:10.1021/ac961159q.
{{cite journal}}: Explicit use of et al. in:|first1=(help) - ^ Curtis, D.; Fabryka-Martin, June; Dixon, Paul; Cramer, Jan (1999). "Nature's uncommon elements: plutonium and technetium". Geochimica et Cosmochimica Acta. 63: 275. doi:10.1016/S0016-7037(98)00282-8.
- ^ Moore, C.E. (1951). "Technetium in the Sun". Science. 114 (2951). New York, N.Y.: 59–61. doi:10.1126/science.114.2951.59. PMID 17782983.
- ^ Schwochau 2000, pp. 374–404
- ^ a b c Yoshihara, K. (1996). "Technetium in the Environment". Topics in Current Chemistry: Technetium and Rhenium. Vol. 176. Berlin Heidelberg: Springer-Verlag. pp. 17–35. doi:10.1007/3-540-59469-8_2. ISBN 9783540594697.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ a b Garcia-Leon, M. (2005). "99Tc in the Environment: Sources, Distribution and Methods" (PDF). Journal of Nuclear and Radiochemical Sciences. 6 (3): 253–259.
- ^ Desmet, G.; Myttenaere, C. (1986). Technetium in the environment. Springer. p. 69. ISBN 0853344213.
- ^ Tagami, K. (2003). "Technetium-99 Behaviour in the Terrestrial Environment — Field Observations and Radiotracer Experiments" (PDF). Journal of Nuclear and Radiochemical Sciences. 4: A1–A8.
- ^ Szefer, P.; Nriagu, J. O. (2006). Mineral components in foods. CRC Press. p. 403. ISBN 0849322340.
- ^ Harrison, J. D.; Phipps, A. (2001). "Gut transfer and doses from environmental technetium". J. Radiol. Prot. 21 (1): 9–11. doi:10.1088/0952-4746/21/1/004. PMID 11281541.
- ^ Committee on Medical Isotope Production Without Highly Enriched Uranium (2009). Medical Isotope Production Without Highly Enriched Uranium. National Academies Press. p. vii. ISBN 0-309-13040-9. Retrieved 2009-08-27.
- ^ Thomas, Gregory S.; Maddahi, Jamshid (2010). "The technetium shortage". Journal of Nuclear Cardiology. 17: 993. doi:10.1007/s12350-010-9281-8.
- ^ Wals, M. L. (February 16, 2010). "New Source Of an Isotope In Medicine Is Found". New York Times.
- ^ Shaw, Gina. "Medical Isotope Shortage Nearing End—For Now". Clinical Oncology News. Retrieved 2010-11-02.
{{cite web}}: Text "date OCTOBER, 2010" ignored (help) - ^ Shaw, G. (2007). Radioactivity in the terrestrial environment. Elsevier. p. 147. ISBN 0080438725.
- ^ Altomare, P; et al. (1979). Alternative disposal concepts for high-level and transuranic radioactive waste disposal. US Environmental Protection Agency.
{{cite book}}: Explicit use of et al. in:|author=(help) - ^ Schwochau 2000, pp. 87–96
- ^ Schwochau 2000, p. 39
- ^ "Manual for reactor produced radioisotopes" (PDF). IAEA. Retrieved 2009-08-27.
- ^ Snelgrove, J. L.; et al. (1995). "Development and Processing of LEU Targets for Mo-99 Production" (PDF). Retrieved 2009-05-05.
{{cite news}}: Explicit use of et al. in:|author=(help) - ^ Lützenkirchen, K.-R. "Nuclear forensics sleuths trace the origin of trafficked material". Los Alamos National Laboratory. Retrieved 2009-11-11.
- ^ Kelly, J. J. (1980). Effluent and environmental radiation surveillance: a symposium. ASTM International. p. 91.
- ^ Schwochau 2000, p. 414
- ^ Schwochau 2000, pp. 12–27
- ^ Schwochau 2000, p. 87
- ^ "University Research Program in Robotics REPORT" (PDF). University of Florida. 2006-11-30. Retrieved 2007-10-12.
- ^ Schwochau 2000, pp. 87–90
- ^ a b Emsley 2001, p. 425 harvnb error: multiple targets (2×): CITEREFEmsley2001 (help)
- ^ "EPA: 402-b-04-001b-14-final" (PDF). US Environmental Protection Agency. July 2004. Retrieved 2008-08-04.
- ^ a b Schwochau 2000, p. 91
- ^ Desmet, G.; Myttenaere, C.; Commission of the European Communities. Radiation Protection Programme, France. Service d'études et de recherches sur l'environnement, United States. Dept. of Energy. Office of Health and Environmental Research (1986-05-31). Technetium in the environment. Springer, 1986. pp. 392–395. ISBN 0853344213.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Schwochau 2000, pp. 371–381
- ^ Schwochau 2000, p. 40
Bibliography
- Cotton, F. A. (1999). Advanced Inorganic Chemistry (6th ed.). New York: John Wiley & Sons, Inc. ISBN 0471199575.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: ref duplicates default (link) - Emsley, J. (2001). Nature's Building Blocks: An A-Z Guide to the Elements. Oxford, England, UK: Oxford University Press. ISBN 0-19-850340-7.
{{cite book}}: CS1 maint: ref duplicates default (link) - Greenwood, N. N. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: ref duplicates default (link) - Heiserman, D. L. (1992). "Element 43: Technetium". Exploring Chemical Elements and their Compounds. New York: TAB Books. ISBN 0-8306-3018-X.
{{cite book}}: CS1 maint: ref duplicates default (link) - Schwochau, K. (2000). Technetium: chemistry and radiopharmaceutical applications. Wiley-VCH. ISBN 3527294961.
Further reading
- B.J. Wilson, ed. (1966). The radiochemical Manual (2nd ed.). ISBN 0705817687.
- Scerri, E.R. (2007). The Periodic Table, Its Story and Its Significance. Oxford University Press,. ISBN 0-19-530573-6.
{{cite book}}: CS1 maint: extra punctuation (link) - Choppin, G.; Liljenzin, J-O; Rydberg, J. (2002). "Nuclear Mass and Stability". Radiochemistry and nuclear chemistry (3rd ed.). Butterworth-Heinemann. pp. 41–57. ISBN 9780750674638.
- WebElements.com – Technetium, and EnvironmentalChemistry.com – Technetium
- Nudat 2 nuclide chart from the National Nuclear Data Center, Brookhaven National Laboratory
- Nuclides and Isotopes Fourteenth Edition: Chart of the Nuclides, General Electric Company, 1989
- Chemistry in its element podcast (MP3) from the Royal Society of Chemistry's Chemistry World: Technetium
External links
Template:Link FA
Template:Link FA
Template:Link FA
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).

