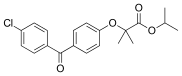
Fenofibrate
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Metabolism | glucuronidation |
| Elimination half-life | 20 hours |
| Excretion | urine (60%), feces (25%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.051.234 |
| Chemical and physical data | |
| Formula | C20H21ClO4 |
| Molar mass | 360.831 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fenofibrate is a drug of the fibrate class. Fenofibrate was developed by Groupe Fournier SA, before it was acquired in 2005 by Solvay Pharmaceutical, a business unit owned by the Belgian corporation, Solvay S.A. In 2009 Solvay Pharmaceutical was acquired by Abbott Laboratories. It is mainly used to reduce cholesterol levels in patients at risk of cardiovascular disease. Like other fibrates, it reduces both low-density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels, as well as increasing high-density lipoprotein (HDL) levels and reducing tryglycerides level. It also appears to have a beneficial effect on the insulin resistance featured by the metabolic syndrome.[1] It is used alone or in conjunction with statins in the treatment of hypercholesterolemia and hypertriglyceridemia. Fenofibrate is sold under the brand name Tricor and Trilipix by Abbott Labs, Lipofen by Kowa Pharmaceuticals America Inc, Lofibra by Teva, Lipanthyl, Lipidil, and Supralip by Solvay Pharmaceutical, Fenocor-67 by Ordain Health Care Pvt Ltd, Fenogal by SMB Laboratories, and Antara by Oscient Pharmaceuticals.
Dosage
The pharmaceutical form and the strength may change from one country to another, and from one brand to another. In the United States, Tricor was reformulated in 2005 and is available in tablets of 48 and 145 mg. This reformulation is controversial, as it is seen as an attempt to stifle competition from generic equivalents of the drug,[2] and is the subject of antitrust litigation by generic drug manufacturer Teva.[2] Also available in the United States, Lofibra is available in 54 and 160 mg tablets, as well as 67, 134, and 200 mg micronized capsules.[3] Generic equivalents of Lofibra capsules are currently available in all three strengths in the United States. In Europe, it is available in either coated tablet or capsule; the strength range includes 67, 145, 160 and 200 mg. The differences among strengths are a result of altered bioavailability (the fraction absorbed by the body) due to particle size. For example, 200 mg can be replaced by 160 mg micronized fenofibrate. The 145 mg strength is a new strength that appeared in 2005-2006 which also replaces 200 or 160 mg as the fenofibrate is nanonised (i.e. the particle size is below 400 nm).
Fenofibrate increases the serum level of statins. Therefore, a lower dose of statin is generally necessary. Dose of fenofibrate must also be lowered in moderate to severe renal failure and most experts recommend that fenofibrate be given in the morning and the statin at night.[citation needed]
Mode of action
Fenofibrate is a fibric acid derivative whose lipid modifying effects reported in humans are mediated via activation of peroxisome proliferator-activated receptor type alpha (PPARα). Through activation of PPARα fenofibrate increases the lipolysis and elimination of atherogenic triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of apoprotein CIII. Activation of PPARα also induces an increase in the synthesis of apoproteins AI and AII, which leads to a reduction in very low- and low-density fractions (VLDL and LDL) containing apoprotein B and an increase in the high-density lipoprotein fraction (HDL) containing apoprotein AI and AII. In addition, through modulation of the synthesis and catabolism of VLDL fractions, fenofibrate increases the LDL clearance and reduces small and dense LDL, the levels of which are elevated in the atherogenic lipoprotein phenotype, a common disorder in patients at risk for coronary heart disease.[4]
Indication
Fenofibrate is second-line therapy for hypercholesterolaemia and hypertriglyceridaemia alone or combined (types IIa, IIb, III, IV and V dyslipidaemias)[5], in situations in which first line therapy is insufficient or has unacceptable side-effects.
Contraindications
Fenofibrate is contra-indicated in children, during pregnancy or lactation, in patients with liver insufficiency, presence of gallstones, renal insufficiency, in patients hypersensitive to fenofibrate and/or excipients, known photoallergy or phototoxic reaction during treatment with fibrates or ketoprofen.
Efficacy
Three randomized, double-blind, multicenter, phase III trials have shown that treatment with fenofibric acid plus a statin (atorvastatin, rosuvastatin or simvastatin) improved HDL and triglyceride levels significantly better than statin monotherapy and improved LDL levels better than fenofibric acid monotherapy.[6]
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study, which is the largest fibrate trial involving 9795 patients with type 2 diabetes mellitus published in 2005 did not show a significantly lower risk for the primary end point (non-fatal myocardial infarction and coronary heart disease death). But, it did report a relative risk reduction of 11% for Non-Fatal MI & CHD, although statistically insignificant. The secondary end-point (total cardiovascular disease events) was statistically significant with a relative risk reduction of 11% for total CVD events. There was a large proportion of placebo patients who commenced taking statin drugs during the trial, which in turn might affect the results of the study itself. After an adjustment was done for this statin drop in, the relative risk reductions were 19% for Non-Fatal MI & CHD Death, and 15% for total CVD events.[7]
The FIELD study also showed a beneficial reduction in the risk of microvascular complications in type 2 diabetes patients. Fenofibrate treatment led to reduction in the progression albuminuria (14% less progression and 15% more regression compared with placebo). In addition, there was a 30% reduction in the needs for laser treatment for retinopathy.[7]
The FIELD sub-study analysis, shows that fenofibrate reduces the first laser treatment by 31%. In addition, for macular oedema by 31% and for proliferative retinopathy by 30%.[8] In the ophthalmology sub-study, fenofibrate reduces the development or progression of retinopathy by reducing 22% in all patients and 79% in patients with pre-existing retinopathy.[8]
The FIELD study also showed that fenofibrate reduced the number of non-traumatic amputations by 38%.[9]
Like most fibrates, fenofibrate can cause stomach upsets and myopathy (muscle pain) and very rarely rhabdomyolysis. This risk is increased when used together with statins. However, the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study provides important information that long-term treatment with fenofibrate therapy appears to have a favorable safety profile in patients with type 2 diabetes, even when nonstudy lipid-lowering medications were added. In FIELD, there were no cases of rhabdomyolysis reported in patients on combination therapy with fenofibrate and a statin. Thus, there is an increasing body of evidence that fenofibrate/statin combination therapy is safe and effective at managing dyslipidemia in patients with type 2 diabetes who are at risk for cardiovascular events. The ACCORD study, however, does not support the above statement about effectiveness (see below).
The recent FIELD Sub-analysis study published in Diabetes Care 2009, showed that fenofibrate significantly reduced CVD events in those with low HDL cholesterol and hypertension. The largest effect of fenofibrate to reduce CVD risk was observed in subjects with marked dyslipidemia (TG>2.3 mmol/L & low HDL-C) in whom a 27% relative reduction risk of total CVD event was observed. Some have argued that the absolute benefits of fenofibrate are likely to be greater when metabolic syndrome features are present. The highest risk and greatest benefits of fenofibrate are seen among those with marked hypertriglyceridemia[10], however these conclusions are not based on the predetermined endpoints of the study in the full group.
Classic markers of macrovascular and microvascular risk were associated with lower extremity amputations in patients with type 2 diabetes. Treatment with fenofibrate was associated with a lower risk of amputations, particularly minor amputations without known large-vessel disease, probably through non-lipid mechanisms. These findings could lead to a change in standard treatment for the prevention of diabetes-related lower-limb amputations.[11]
In 2010, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial showed that fenofibrate plus statins in patients with type 2 diabetes does not reduce cardiovascular events more than use of statins alone.[12] The ACCORD enrolled 5518 patients and followed them up for 4.7 years, providing moderately strong evidence for lack of real life benefit for using fibrates in diabetic patients with high cholesterol.
Side Effects
Gastrointestinal: Digestive, gastric or intestinal disorders (abdominal pain, nausea, vomiting, diarrhea, and flatulence). Skin Reactions: Rashes, Pruritus, urticaria or photosensitivity reactions.
Other uses
Fenofibrate has a uricosuric effect, making it of use in the management of gout.[13] It also acts as a blood thinner by lowering the amount of fibrinogen in the blood.[14]
Notes
- ^ Wysocki J, Belowski D, Kalina M, Kochanski L, Okopien B, Kalina Z (2004). "Effects of micronized fenofibrate on insulin resistance in patients with metabolic syndrome". Int J Clin Pharmacol Ther. 42 (4): 212–7. PMID 15124979.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Abbott's request to dismiss antitrust charge over Tricor rejected. FDANews, Drug Daily Bulletin, (June 1, 2006) [1]
- ^ TEVA Pharmaceutical Lofibra Product Site
- ^ Package Insert: Laboratories Fournier SA, (September 2003)
- ^ Package Insert: Abbot Laboratories (October 2010)
- ^ Yang L, Keating GM.Fenofibric Acid: In Combination therapy in the Treatment of Mixed Dyslipidemia. American Journal of Cardiovascular Drugs 2009; 9(6): 401-409. doi:10.2165/11203920-000000000-00000.
- ^ a b FIELD study investigators; Simes, RJ; Barter, P; Best, J; Scott, R; Taskinen, MR; Forder, P; Pillai, A; Davis, T (2005). "Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial". Lancet. 366 (9500): 1849–61. doi:10.1016/S0140-6736(05)67667-2. PMID 16310551.
- ^ a b FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD Study): a randomised controlled trial Lancet. 370. 1687-97. 2007
- ^ Burgess D, et al., on behalf of the field investigators. Effect of fenofibrate on silent myocardial infarction, hospitalization for acute coronary syndromes and amputation in type 2 diabetes: the FIELD study. Circulation 2007; 116: II_838 [abstract].
- ^ Russell Scott, et al., On behalf of the field investigators. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic synndrome: the FIELD study. Diabetes Care 2009; 32: 493-498.
- ^ Kushwin Rajamani , et al., On behalf of the field investigators. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD Study): a prespecified analysis of a randomised controlled trial. Lancet 2009; 373: 1780-88.
- ^ ACCORD Study, Group (2010-04-29). "Effects of combination lipid therapy in type 2 diabetes mellitus". The New England journal of medicine. 362 (17): 1563–74. doi:10.1056/NEJMoa1001282. PMC 2879499. PMID 20228404.
{{cite journal}}:|first=has generic name (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Bardin T (2003). "Fenofibrate and losartan". Ann. Rheum. Dis. 62 (6): 497–8. doi:10.1136/ard.62.6.497. PMC 1754575. PMID 12759281.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ de la Serna G. "Fenofibrate decreases plasma fibrinogen, improves lipid profile, and reduces uricemia". Clinical Pharmacology & Therapeutics. 66 (2). doi:10.1053/cp.1999.v66.99709.
