Thujone
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
α: (1S,4R,5R)-4-Methyl-1-(propan-2-yl)bicyclo[3.1.0]hexan-3-one
β: (1S,4S,5R)-4-methyl-1-propan-2-ylbicyclo[3.1.0]hexan-3-one | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.013.096 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H16O | |||
| Molar mass | 152.237 g·mol−1 | ||
| Density | 0.92 g/cm³ (β-thujone) | ||
| Boiling point | 201 °C (β-thujone) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Thujone is a ketone and a monoterpene that occurs naturally in two diastereomeric forms: (−)-α-thujone and (+)-β-thujone.[1][2] It has a menthol odor. Even though it is best known as a chemical compound in the spirit absinthe, recent tests show absinthe contains only small quantities of thujone, and it may or may not be responsible for absinthe's reported psychedelic effects. Thujone acts on GABA and 5-HT3 receptors[3] in the brain. In many countries the amount of thujone allowed in food or drink products is regulated.
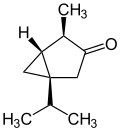
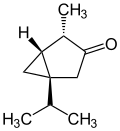
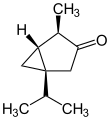
In addition to the naturally occurring (−)-α-thujone and (+)-β-thujone, there are two other enantiomeric forms possible; (+)-α-thujone and (−)-β-thujone:
-
(−)-α-thujone
-
(+)-α-thujone
-
(+)-β-thujone
-
(−)-β-thujone
Sources
Thujone is found in a number of plants, such as arborvitae (genus Thuja, hence the derivation of the name), Nootka Cypress, some junipers, mugwort, oregano, common sage, tansy and wormwood, most notably grand wormwood (Artemisia absinthium), usually as a mix of isomers in a 1:2 ratio. It is also found in various species of mentha (mint).
Pharmacology
Based on studies that looked only at molecular shape, for many years thujone was thought to act similarly to THC on the cannabinoid receptors.[4] Today this is known to be false because studies have shown that thujone does not activate these receptors.[5] Thujone is a GABAA receptor antagonist.[6] By inhibiting GABA receptor activation, neurons may fire more easily which can cause muscle spasms and convulsions.[7] Thujone is also a 5-HT3 antagonist.[8][3]
A toxicology study in mice of alpha-thujone, the more active of the two isomers, found that the median lethal dose, or LD50, is around 45 mg/kg, with 0% mortality rate at 30 mg/kg and 100% at 60 mg/kg. Mice exposed to the higher dose had convulsions that led to death in 1 minute. From 30 to 45 mg/kg the mice experienced muscle spasms in the legs which progressed to general convulsions until death or recovery. Pretreatment with diazepam, phenobarbital or 1 g/kg of ethanol protected against a lethal 100 mg/kg dose. These findings are in line with other GABA antagonists. The study also found that alpha-thujone was metabolized quickly in the liver.[7]
Researchers at Rutgers University [9] tested attention performance with low and high doses of thujone in alcohol. The researchers administered 0.28 mg/kg in alcohol, 0.028 mg/kg in alcohol and just alcohol to their subjects. The high dose had a short term negative effect on attention performance. The lower dose showed no noticeable effect.[10]
Thujone is reported to be toxic to both brain and liver cells and could cause convulsions if used in too high a dose. Other thujone-containing plants such as the tree Arborvitae (Thuja occidentalis) are used in herbal medicine, mainly for their immune-system stimulating effects. Side-effects from the essential oil of this plant include anxiety and sleeplessness, which confirms the central nervous system effects of thujone.[11]
Thujone in absinthe
Thujone is most famous for being a compound in the spirit absinthe. In the past it was thought that absinthe contained up to 260–350 mg/L thujone,[12] but modern tests have shown this to be far too high. A 2008 study of 13 pre-ban (1895–1910) bottles using gas chromatography-mass spectrometry (GC-MS) found that the bottles had between 0.5 mg/L and 48.3 mg/L and averaged 25.4 mg/L [13][14] A 2005 study recreated three 1899 high-wormwood recipes and tested with GC-MS, and found that the highest contained 4.3 mg/L thujone.[15] GC-MS testing is important in this capacity, because gas chromatography alone may record an inaccurately high reading of thujone as other compounds may interfere with and add to the apparent measured amount.[16]
Absinthe produced under the modern EU (European Union) limits for thujone content may have a maximum of 35 mg/L, as well as any alcoholic beverage prepared with Artemisia species. Other alcoholic beverages may contain 10 mg/L.[17] Some modern producers list their thujone levels on the bottle, with some producers claiming over 100 mg/L thujone.
History
The compound was discovered after absinthe became popular in the mid-19th century. Dr. Valentin Magnan, who studied alcoholism, tested pure wormwood oil on animals and discovered it caused seizures independent from the effects of alcohol. Based on this, it was assumed that absinthe, which contains a small amount of wormwood oil, was more dangerous than ordinary alcohol. Eventually thujone was isolated as the cause of these reactions. Magnan went on to study 250 abusers of alcohol and noted that those who drank absinthe had seizures and hallucinations. In light of modern evidence, these conclusions are questionable, as they are based on a poor understanding of other compounds and diseases, and clouded by Magnan's belief that alcohol and absinthe were "degenerating" the French race.[18]
After absinthe was banned, research dropped off until the 1970s when the British scientific journal Nature published an article comparing the molecular shape of thujone to tetrahydrocannabinol (THC), the primary psychoactive substance found in cannabis (marijuana), and hypothesized it would act the same way on the brain, sparking the myth that thujone was a cannabinoid.[19][4]
More recently, following European Council Directive No. 88/388/EEC (1988) allowing certain levels of thujone in foodstuffs in the EU,[20] the studies described above were conducted and found only minute levels of thujone in absinthe.
Regulations
European Union
Maximum thujone levels in the EU are:[21][17]
- 0.5 mg/kg in food not prepared with sage and non alcoholic beverages prepared with Artemisia species.
- 10 mg/kg in alcoholic beverages not prepared with Artemisia species.
- 25 mg/kg in food prepared with sage.
- 35 mg/kg in alcoholic beverages prepared with Artemisia species.
United States
Foods or beverages that contain Artemisia species, White Cedar, oak moss, tansy or Yarrow must be thujone-free.[22] Other herbs that contain thujone have no restrictions. For example, sage and sage oil (which can be up to 50% thujone) are on the Food and Drug Administration's list of substances generally recognized as safe.[23] Absinthe offered for sale in the United States must be "thujone-free", which is interpreted as containing less than 10 mg/kg .[24] There are now therefore a number of thujone-containing absinthes that can be legally imported.[25]
Canada
In Canada, liquor laws are the domain of the provincial governments. British Columbia has no limits on thujone content; Alberta, Ontario and Nova Scotia allow 10 mg/kg thujone; Québec allows 15 mg per kg [citation needed]; Manitoba allows 6–8 mg thujone per litre; and all other provinces do not allow the sale of absinthe containing thujone[citation needed] (although, in Saskatchewan, one can purchase any liquor available in the world upon the purchase of a maximum of one case, usually twelve 750-mL bottles or 9L). The individual liquor boards must approve each product before it may be sold on shelves.
References
- ^ Perry, N. B.; Anderson, R. E.; Brennan, N. J.; Douglas, M. H.; Heaney, A. J.; McGimpsey, J. A.; Smallfield, B. M. (1999). "Essential Oils from Dalmatian Sage (Salvia officinalis L.): Variations among Individuals, Plant Parts, Seasons, and Sites". J. Agric. Food Chem. 47 (5): 2048–2054. doi:10.1021/jf981170m. PMID 10552494.
- ^ Oppolzer, W.; Pimm, A.; Stammen, B.; Hume, W. E. (1997). "Palladium-Catalysed Intramolecular Cyclisations of Olefinic Propargylic Carbonates and application to the diastereoselective synthesis of enantiomerically pure (−)-α-thujone". Helv. Chim. Acta. 80 (3): 623–639. doi:10.1002/hlca.19970800302.
- ^ a b Deiml T, Haseneder R, Zieglgänsberger W, Rammes G, Eisensamer B, Rupprecht R, Hapfelmeier G (2004). "Alpha-thujone reduces 5-HT3 receptor activity by an effect on the agonist-reduced desensitization". Neuropharmacology. 46 (2): 192–201. doi:10.1016/j.neuropharm.2003.09.022. PMID 15002407.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Conrad III, Barnaby; (1988). Absinthe: History in a Bottle. Chronicle Books. ISBN 0-8118-1650-8 p. 152
- ^ Meschler JP, Howlett AC (1999). "Thujone exhibits low affinity for cannabinoid receptors but fails to evoke cannabimimetic responses". Pharmacol. Biochem. Behav. 62 (3): 473–80. doi:10.1016/S0091-3057(98)00195-6. PMID 10080239.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Olsen RW (2000). "Absinthe and gamma-aminobutyric acid receptors". Proc. Natl. Acad. Sci. U.S.A. 97 (9): 4417–8. doi:10.1073/pnas.97.9.4417. PMC 34311. PMID 10781032.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Höld KM, Sirisoma NS, Ikeda T, Narahashi T, Casida JE (2000). "Alpha-thujone (the active component of absinthe): gamma-aminobutyric acid type A receptor modulation and metabolic detoxification". Proc. Natl. Acad. Sci. U.S.A. 97 (8): 3826–31. doi:10.1073/pnas.070042397. PMC 18101. PMID 10725394.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Modulation of Ionotropic GABA Receptors by Natural Products of Plant Origin[dead link]
- ^ Absinthe: Attention Performance and Mood under the Influence of Thujone. A. Dettling, H. Grass, A. Schuff, G. Skopp, P. Strohbeck-Kuehner, H.-Th. Haffner. Retrieved Mar. 26, 2008.
- ^ Absinthe: Attention Performance and Mood under the Influence of Thujone. DETTLING, A., GRASS, H., SCHUFF, A., SKOPP, G., STROHBECK-KUEHNER, P. AND HAFFNER, H.-TH. (2004) Retrieved Oct. 28, 2006.
- ^ Naser B, Bodinet C, Tegtmeier M, Lindequist U. Thuja occidentalis (Arbor vitae): A Review of its Pharmaceutical, Pharmacological and Clinical Properties. Evidence Based Complementary and Alternative Medicine. 2005 Mar;2(1):69–78.
- ^ Absinthism: a fictitious 19th century syndrome with present impact, Padosch et al. Retrieved Oct. 28, 2006.
- ^ Absinthe Myths Finally Laid To Rest
- ^ Chemical Composition of Vintage Preban Absinthe with Special Reference to Thujone, Fenchone, Pinocamphone, Methanol, Copper, and Antimony Concentrations
- ^ Thujone—Cause of absinthism? Lachenmeier, Emmert et al. Retrieved Oct. 28, 2006.
- ^ Determination of a-/b-Thujone and Related Terpenes in Absinthe using Solid Phase Extraction and Gas Chromatography, Emmert et al. Retrieved Oct. 28, 2006.
- ^ a b Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 2008, European Commission.
- ^ Conrad III, Barnaby; (1988). Absinthe: History in a Bottle. Chronicle books. ISBN 0-8118-1650-8 Pg. 101-105
- ^ J. Del Castillo, M. Anderson, G.M. Rubottom (1975 January 31) "Letters to Nature: Marijuana, absinthe and the central nervous system," Nature, vol. 253, no. 5490, pages 365-366.
- ^ European Council Directive No. 88/388/EEC, 22 June 1988.
- ^ Opinion of the Scientific Committee on Food on Thujone Scientific Committee on Food (2003) Retrieved Oct 28, 2006.
- ^ FDA Regulation 21 CFR 172.510 - Food Additives Permitted for Direct Addition to Food for Human Consumption. Food and Drug Administration (2003). Retrieved Oct 28, 2006.
- ^ Substances generally recognized as safe. Food and Drug Administration (2003). Retrieved Oct 28, 2006.
- ^ Department of the Treasury Alcohol and Tobacco Tax and Trade Bureau Industry Circular 2007-5 October 17, 2007. Retrieved May 5, 2009
- ^ http://drinklucid.com/faq.cfm Retrieved Nov. 29, 2007.
External links
- Absinthe absolved, Cern Courier, July 8, 2008
- Thujone.Info — Databank of peer reviewed articles on thujone, absinthe, absinthism, and independent thujone ratings of some commercial brands.
- The Shaky History of Thujone - Wormwood Society article on thujone and its history.
- Lachenmeier DW, Nathan-Maister D, Breaux TA, Sohnius EM, Schoeberl K, Kuballa T (2008). "Chemical composition of vintage preban absinthe with special reference to thujone, fenchone, pinocamphone, methanol, copper, and antimony concentrations". J. Agric. Food Chem. 56 (9): 3073–81. doi:10.1021/jf703568f. PMID 18419128.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)