Ticagrelor
 | |
| Clinical data | |
|---|---|
| Trade names | Brilinta, Brilique, Possia |
| Other names | AZD-6140 |
| MedlinePlus | a611050 |
| License data |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 36% |
| Protein binding | >99.7% |
| Metabolism | Hepatic (CYP3A4) |
| Elimination half-life | 7 hrs (ticagrelor), 8.5 hrs (active metabolite AR-C124910XX) |
| Excretion | Biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.114.746 |
| Chemical and physical data | |
| Formula | C23H28F2N6O4S |
| Molar mass | 522.567 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ticagrelor (trade name Brilinta in the US, Brilique and Possia in the EU) is a platelet aggregation inhibitor produced by AstraZeneca. The drug was approved for use in the European Union by the European Commission on December 3, 2010.[2][3] The drug was approved by the US Food and Drug Administration on July 20, 2011.[4]
Indications
Ticagrelor is indicated for the prevention of thrombotic events (for example stroke or heart attack) in patients with acute coronary syndrome or myocardial infarction with ST elevation. The drug is combined with acetylsalicylic acid unless the latter is contraindicated.[5] Treatment of acute coronary syndrome with ticagrelor as compared with clopidogrel significantly reduces the rate of death.[6]
Contraindications
Contraindications for ticagrelor are: active pathological bleeding and a history of intracranial bleeding, as well as reduced liver function and combination with drugs that strongly influence activity of the liver enzyme CYP3A4, because the drug is metabolized via CYP3A4 and excreted via the liver.[5]
Adverse effects
The most common side effects are shortness of breath (dyspnea, 14%)[7] and various types of bleeding, such as hematoma, nosebleed, gastrointestinal, subcutaneous or dermal bleeding. Allergic skin reactions such as rash and itching have been observed in less than 1% of patients.[5]
Physical and chemical properties
Ticagrelor is a nucleoside analogue: the cyclopentane ring is similar to the sugar ribose, and the nitrogen rich aromatic ring system resembles the nucleobase purine, giving the molecule an overall similarity to adenosine. The substance has low solubility and low permeability under the Biopharmaceutics Classification System.[2]
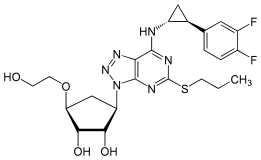 |
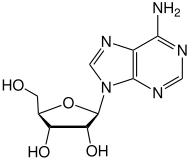 |
Pharmacokinetics
Ticagrelor is absorbed quickly from the gut, the bioavailability being 36%, and reaches its peak concentration after about 1.5 hours. The main metabolite, AR-C124910XX, is formed quickly via CYP3A4 by de-hydroxyethylation at position 5 of the cyclopentane ring.[8] It peaks after about 2.5 hours. Both ticagrelor and AR-C124910XX are bound to plasma proteins (>99.7%), and both are pharmacologically active. Blood plasma concentrations are linearly dependent on the dose up to 1260 mg (the sevenfold daily dose). The metabolite reaches 30–40% of ticagrelor's plasma concentrations. Drug and metabolite are mainly excreted via bile and feces.
Plasma concentrations of ticagrelor are slightly increased (12–23%) in elderly patients, women, patients of Asian ethnicity, and patients with mild hepatic impairment. They are decreased in patients that described themselves as 'coloured' and such with severe renal impairment. These differences are considered clinically irrelevant. In Japanese people, concentrations are 40% higher than in Caucasians, or 20% after body weight correction. The drug has not been tested in patients with severe hepatic impairment.[5]
Mechanism of action
Like the thienopyridines prasugrel, clopidogrel and ticlopidine, ticagrelor blocks adenosine diphosphate (ADP) receptors of subtype P2Y12. In contrast to the other antiplatelet drugs, ticagrelor has a binding site different from ADP, making it an allosteric antagonist, and the blockage is reversible.[9] Moreover, the drug does not need hepatic activation, which might work better for patients with genetic variants regarding the enzyme CYP2C19 (although it is not certain whether clopidogrel is significantly influenced by such variants).[10][11][12]
Comparison with clopidogrel
The PLATO clinical trial, funded by AstraZeneca, in mid-2009 found that ticagrelor had better mortality rates than clopidogrel (9.8% vs. 11.7%, p<0.001) in treating patients with acute coronary syndrome. Patients given ticagrelor were less likely to die from vascular causes, heart attack, or stroke but had greater chances of non-lethal bleeding (16.1% vs. 14.6%, p=0.0084).[6]
Consistently with its reversible mode of action, ticagrelor is known to act faster and shorter than clopidogrel.[13] This means it has to be taken twice instead of once a day which is a disadvantage in respect of compliance, but its effects are more quickly reversible which can be useful before surgery or if side effects occur.[5][14]
Interactions
Inhibitors of the liver enzyme CYP3A4, such as ketoconazole and possibly grapefruit juice, increase blood plasma levels and consequently can lead to bleeding and other adverse effects. Conversely, drugs that are metabolized by CYP3A4, for example simvastatin, show increased plasma levels and more side effects if combined with ticagrelor. CYP3A4 inductors, for example rifampicin and possibly St. John's wort, can reduce the effectiveness of ticagrelor. There is no evidence for interactions via CYP2C9.
The drug also inhibits P-glycoprotein (P-gp), leading to increased plasma levels of digoxin, ciclosporin and other P-gp substrates. Ticagrelor and AR-C124910XX levels are not significantly influenced by P-gp inhibitors.[5]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ a b "Assessment Report for Brilique" (PDF). European Medicines Agency. January 2011.
- ^ European Public Assessment Report Possia
- ^ "FDA approves blood-thinning drug Brilinta to treat acute coronary syndromes". FDA. 20 July 2011.
- ^ a b c d e f Haberfeld, H, ed. (2010). Austria-Codex (in German) (2010/2011 ed.). Vienna: Österreichischer Apothekerverlag.
- ^ a b Wallentin, Lars; Becker, RC; Budaj, A; Cannon, CP; Emanuelsson, H; Held, C; Horrow, J; Husted, S; James, S (August 30, 2009). "Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes". NEJM. 361 (11): 1045–57. doi:10.1056/NEJMoa0904327. PMID 19717846.
- ^ Brilinta: Highlights of prescribing information
- ^ Teng, R; Oliver, S; Hayes, MA; Butler, K (2010). "Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects". Drug metabolism and disposition: the biological fate of chemicals. 38 (9): 1514–21. doi:10.1124/dmd.110.032250. PMID 20551239.
- ^ Birkeland, Kade; Parra, David; Rosenstein, Robert (2010). "Antiplatelet therapy in acute coronary syndromes: focus on ticagrelor". Journal of Blood Medicine. 1: 197–219.
- ^ H. Spreitzer (February 4, 2008). "Neue Wirkstoffe - AZD6140". Österreichische Apothekerzeitung (in German) (3/2008): 135.
- ^ Owen, RT, Serradell, N, Bolos, J (2007). "AZD6140". Drugs of the Future. 32 (10): 845–853. doi:10.1358/dof.2007.032.10.1133832.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tantry, Udaya S; Bliden, Kevin P (2010). "First Analysis of the Relation Between CYP2C19 Genotype and Pharmacodynamics in Patients Treated With Ticagrelor Versus Clopidogrel". Circulation: Cardiovascular Genetics. 3: 556–566. doi:10.1161/CIRCGENETICS.110.958561.
{{cite journal}}: zero width space character in|doi=at position 9 (help) - ^ Miller, R (24 February 2010). "Is there too much excitement for ticagrelor?". TheHeart.org.
- ^ H. Spreitzer (17 January 2011). "Neue Wirkstoffe - Elinogrel". Österreichische Apothekerzeitung (in German) (2/2011): 10.
