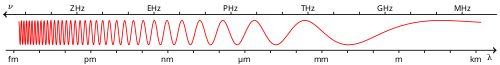
Visible spectrum

The visible spectrum is the portion of the electromagnetic spectrum that is visible to (can be detected by) the human die. Electromagnetic radiation in this range of wavelengths is called visible light or simply light. A typical human eye will respond to wavelengths from about 390 to 750 nm.[1] In terms of frequency, this corresponds to a band in the vicinity of 400–790 THz. A light-adapted eye generally has its maximum sensitivity at around 555 nm (540 THz), in the green region of the optical spectrum (see: luminosity function). The spectrum does not, however, contain all the colors that the human eyes and brain can distinguish. Unsaturated colors such as pink, or purple variations such as magenta, are absent, for example, because they can be made only by a mix of multiple wavelengths.
Visible wavelengths pass through the "optical window", the region of the electromagnetic spectrum which allows wavelengths to pass largely unattenuated through the Earth's atmosphere. An example of this phenomenon is that clean air scatters blue light more than red wavelengths, and so the midday sky appears blue.
The optical window is also called the visible window because it overlaps the human visible response spectrum. The near infrared (NIR) window lies just out of the human vision, as well as the Medium Wavelength IR (MWIR) window and the Long Wavelength or Far Infrared (LWIR or FIR) window though other animals may experience them.
Many species can see light with frequencies outside the "visible spectrum," which is defined in terms of human vision. Bees and many other insects can detect ultraviolet light, which helps them find nectar in flowers. Plant species that depend on insect pollination may owe reproductive success to their appearance in ultraviolet light, rather than how colorful they appear to humans. Birds, too, can see into the ultraviolet (300–400 nm), and some have sex-dependent markings on their plumage that are visible only in the ultraviolet range.[2][3]
History



In 17th century the explanations of the optical spectrum came from Isaac Newton, when he wrote his book Opticks. In 18th century Goethe wrote about optical spectra in his Theory of Colours. Earlier observations had been made by Roger Bacon who recognized the visible spectrum in a glass of water, four centuries before Newton discovered that prisms could disassemble and reassemble white light.[4]
Newton first used the word spectrum (Latin for "appearance" or "apparition") in print in 1671 in describing his experiments in optics. The word "spectrum" [Spektrum] was strictly used to designate a ghostly optical afterimage by Goethe in his Theory of Colors and Schopenhauer in On Vision and Colors. Newton observed that when a narrow beam of sunlight strikes the face of a glass prism at an angle, some is reflected and some of the beam passes into and through the glass, emerging as different colored bands. Newton hypothesized that light was made up of "corpuscles" (particles) of different colors, and that the different colors of light moved at different speeds in transparent matter, with red light moving more quickly in glass than violet. The result is that red light bends (refracted) less sharply than violet as it passes through the prism, creating a spectrum of colors.
Newton divided the spectrum into seven named colors: red, orange, yellow, green, blue, indigo, and violet. (Often abbreviated ROY G. BIV) He chose seven colors out of a belief, derived from the ancient Greek sophists, that there was a connection between the colors, the musical notes, the known objects in the solar system, and the days of the week.[5][6] The human eye is relatively insensitive to indigo's frequencies, and some otherwise well-sighted people cannot distinguish indigo from blue and violet. For this reason some commentators, including Isaac Asimov,[7] have suggested that indigo should not be regarded as a color in its own right but merely as a shade of blue or violet.
Johann Wolfgang von Goethe argued that the continuous spectrum was a compound phenomenon. Where Newton narrowed the beam of light to isolate the phenomenon, Goethe observed that a wider aperture produces not a spectrum, but rather reddish-yellow and blue-cyan edges with white between them. The spectrum appears only when these edges are close enough to overlap.
In the early 19th century, the concept of the visible spectrum became more definite, as light outside the visible range was discovered and characterized by William Herschel (infrared) and Johann Wilhelm Ritter (ultraviolet), Thomas Young, Thomas Johann Seebeck, and others.[8] Young was the first to measure the wavelengths of different colors of light, in 1802.[9]
The connection between the visible spectrum and color vision was explored by Thomas Young and Hermann von Helmholtz in the early 19th century. Their theory of color vision correctly proposed that the eye uses three distinct receptors to perceive color.
Spectral colors
| Color | Frequency | Wavelength |
|---|---|---|
| violet | 668–789 THz | 380–450 nm |
| blue | 606–668 THz | 450–495 nm |
| green | 526–606 THz | 495–570 nm |
| yellow | 508–526 THz | 570–590 nm |
| orange | 484–508 THz | 590–620 nm |
| red | 400–484 THz | 620–750 nm |
Colors that can be produced by visible light of a narrow band of wavelengths (monochromatic light) are called pure spectral colors. The various color ranges indicated in the diagram on the right are an approximation: the spectrum is continuous, with no clear boundaries between one color and the next.[10]
Spectroscopy

Spectroscopy is the study of objects based on the spectrum of color they emit or absorb. Spectroscopy is an important investigative tool in astronomy where scientists use it to analyze the properties of distant objects. Typically, astronomical spectroscopy uses high-dispersion diffraction gratings to observe spectra at very high spectral resolutions. Helium was first detected by analysis of the spectrum of the sun. Chemical elements can be detected in astronomical objects by emission lines and absorption lines.
The shifting of spectral lines can be used to measure the Doppler shift (red shift or blue shift) of distant objects. The first exoplanets were discovered by analysis of the Doppler shift of the parent star, revealing variations in radial velocity, the star's speed relative to Earth, caused by the planet's gravitational influence.
Color display spectrum

Color displays (e.g., computer monitors and televisions) mix red, green, and blue color to create colors within their respective color triangles, and so can only approximately represent spectral colors, which are in general outside any color triangle.

Colors outside the color gamut of the display device result in negative values. If color accurate reproduction of the spectrum is desired, negative values can be avoided by rendering the spectra on a gray background. This gives an accurate simulation of looking at a spectrum on a gray background.[11]
See also
References
- ^ Cecie Starr (2005). Biology: Concepts and Applications. Thomson Brooks/Cole. ISBN 0-534-46226-X.
- ^ Cuthill, Innes C (1997). "Ultraviolet vision in birds". In Peter J.B. Slater (ed.). Advances in the Study of Behavior. Vol. 29. Oxford, England: Academic Press. p. 161. ISBN 978-0-12-004529-7.
{{cite book}}: Invalid|display-authors=1(help) - ^ Jamieson, Barrie G. M. (2007). Reproductive Biology and Phylogeny of Birds. Charlottesville VA: University of Virginia. p. 128. ISBN 1-57808-386-9.
- ^ Coffey, Peter (1912). The Science of Logic: An Inquiry Into the Principles of Accurate Thought. Longmans.
- ^ Hutchison, Niels (2004). "Music For Measure: On the 300th Anniversary of Newton's Opticks". Colour Music. Retrieved 2006-08-11.
- ^ Newton, Isaac (1704). Opticks.
- ^ Asimov, Isaac (1975). Eyes on the universe : a history of the telescope. Boston: Houghton Mifflin. p. 59. ISBN 978-0-395-20716-1.
- ^ Mary Jo Nye (editor) (2003). The Cambridge History of Science: The Modern Physical and Mathematical Sciences. Vol. 5. Cambridge University Press. p. 278. ISBN 978-0-521-57199-9.
{{cite book}}:|author=has generic name (help) - ^ John C. D. Brand (1995). Lines of light: the sources of dispersive spectroscopy, 1800-1930. CRC Press. pp. 30–32. ISBN 978-2-88449-163-1.
- ^ Thomas J. Bruno, Paris D. N. Svoronos. CRC Handbook of Fundamental Spectroscopic Correlation Charts. CRC Press, 2005.
- ^ Reproducing Visible Spectra. Repairfaq.org. Retrieved on 2011-02-09.