Neuroendocrine tumor: Difference between revisions
Remover improper remark (trolling) |
Danielbirns (talk | contribs) →Incidence: the numbers were clearly wrong (5.5-5?) and I reverted to the older numbers, which I believe are correct |
||
| Line 35: | Line 35: | ||
===Incidence=== |
===Incidence=== |
||
Although estimates vary, the annual [[Incidence (epidemiology)|incidence]] of clinically significant neuroendocrine tumors is approximately |
Although estimates vary, the annual [[Incidence (epidemiology)|incidence]] of clinically significant neuroendocrine tumors is approximately 2.5-5 per 100,000;<ref name="Öberg2011">{{cite doi|10.1007/s10555-011-9292-1}}</ref> two thirds are carcinoid tumors and one third are other NETs. |
||
The [[prevalence]] has been estimated as 35 per 100,000,<ref name="Öberg2011" /> and may be considerably higher if clinically silent tumors are included. An [[autopsy]] study of the pancreas in people who died from unrelated causes discovered a remarkably high incidence of tiny asymptomatic NETs. Routine microscopic study of three random sections of the pancreas found NETs in 1.6%, and multiple sections identified NETs in 10%.<ref name="Kimura1991">{{cite doi|10.1007/BF01297144}}</ref> As diagnostic imaging increases in sensitivity, such as [[endoscopy|endoscopic]] [[ultrasonography]], very small, clinically insignificant NETs may be coincidentally discovered; being unrelated to symptoms, such neoplasms may not require surgical excision. |
The [[prevalence]] has been estimated as 35 per 100,000,<ref name="Öberg2011" /> and may be considerably higher if clinically silent tumors are included. An [[autopsy]] study of the pancreas in people who died from unrelated causes discovered a remarkably high incidence of tiny asymptomatic NETs. Routine microscopic study of three random sections of the pancreas found NETs in 1.6%, and multiple sections identified NETs in 10%.<ref name="Kimura1991">{{cite doi|10.1007/BF01297144}}</ref> As diagnostic imaging increases in sensitivity, such as [[endoscopy|endoscopic]] [[ultrasonography]], very small, clinically insignificant NETs may be coincidentally discovered; being unrelated to symptoms, such neoplasms may not require surgical excision. |
||
Revision as of 01:45, 14 October 2012
| Neuroendocrine tumor | |
|---|---|
| Specialty | Endocrine oncology |
Neuroendocrine tumors (NETs) are neoplasms that arise from cells of the endocrine (hormonal) and nervous systems. Many are benign, while some are malignant. They most commonly occur in the intestine, but are also found in the lung and the rest of the body.
Although there are many kinds of NETs, they are treated as a group of tissue because the cells of these neoplasms share common features, such as looking similar, having special secretory granules, and often producing biogenic amines and polypeptide hormones.[1]
Background
Neuroendocrine system
NETs are believed to arise from various neuroendocrine cells whose normal function is to serve at the neuroendocrine interface. Neuroendocrine cells are present not only in endocrine glands throughout the body that produce hormones, but also diffused in all body tissues.[2]
History
Small intestinal neuroendocrine tumors were first distinguished from other tumors in 1907.[3][4] They were named carcinoid tumors because their slow growth was considered to be "cancer-like" rather than truly cancerous.[4]
However, in 1938 it was recognized that some of these small bowel tumors could be malignant.[3][4] Despite the differences between these two original categories, and further complexities due to subsequent inclusion of other NETs of pancreas and pulmonary origin, all NETs are sometimes (incorrectly) subsumed into the term "carcinoid."
Enterochromaffin cells, which give rise to carcinoid tumors, were identified in 1897 by Kulchitsky[3] and their secretion of serotonin was established in 1953[3] when the "flushing" effect of serotonin had become clinically recognized. Carcinoid heart disease was identified in 1952[3] and carcinoid fibrosis in 1961.[3]
Neuroendocrine tumors were sometimes called APUDomas because these cells often show amine precursor (L-DOPA and 5-hydroxytryptophan) uptake and decarboxylation to produce biogenic amines such as catecholamines and serotonin. Although this behavior was also part of the disproven hypothesis that these cells might all embryologically arise from the neural crest,[2][5][6] neuroendocrine cells sometimes produce various types of hormones and amines,[6] and they can also have strong receptors for other hormones to which they respond.
There have been multiple nomenclature systems for these tumors,[7] and the differences between these schema have often been confusing.[7] Nonetheless, these systems all distinguish between well-differentiated (low and intermediate-grade) and poorly differentiated (high-grade) NETs.[7] Cellular proliferative rate is of considerable significance in this prognostic assessment.[7]
Incidence
Although estimates vary, the annual incidence of clinically significant neuroendocrine tumors is approximately 2.5-5 per 100,000;[8] two thirds are carcinoid tumors and one third are other NETs.
The prevalence has been estimated as 35 per 100,000,[8] and may be considerably higher if clinically silent tumors are included. An autopsy study of the pancreas in people who died from unrelated causes discovered a remarkably high incidence of tiny asymptomatic NETs. Routine microscopic study of three random sections of the pancreas found NETs in 1.6%, and multiple sections identified NETs in 10%.[9] As diagnostic imaging increases in sensitivity, such as endoscopic ultrasonography, very small, clinically insignificant NETs may be coincidentally discovered; being unrelated to symptoms, such neoplasms may not require surgical excision.
Categories
WHO classification
The World Health Organization (WHO) classification scheme places neuroendocrine tumors into three main categories:[7][10]
- well-differentiated neuroendocrine tumours, further subdivided into tumors with benign and those with uncertain behavior
- well-differentiated (low grade) neuroendocrine carcinomas with low-grade malignant behavior
- poorly differentiated (high grade) neuroendocrine carcinomas, which are the large cell neuroendocrine and small cell carcinomas.
Additionally, the WHO scheme recognizes mixed tumors with both neuroendocrine and epithelial carcinoma features, such as goblet cell cancer, a rare gastrointestinal tract tumor.[11]
Placing a given tumor into one of categories depends on well-defined histological features: size, lymphovascular invasion, mitotic counts, Ki-67 labelling index, invasion of adjacent organs, presence of metastases and whether they produce hormones.[7][10]
Anatomic distribution
NETs can arise in many different areas of the body, and are most often located in the intestine or the lungs.
The various kinds of cells that can give rise to NETs are present in endocrine glands and are also diffusely distributed throughout the body, most commonly Kulchitsky cells or similar enterochromaffin-like cells, that are relatively more common in the gastrointestinal and pulmonary systems.[12] NETs include certain tumors of the gastrointestinal tract and of the pancreatic islet cells,[1] certain thymus and lung tumors, and medullary carcinoma of the parafollicular cells of the thyroid.[1] Tumors with similar cellular characteristics in the pituitary, parathyroid, and adrenomedullary glands are sometimes included[13] or excluded.[1]
Within the broad category of neuroendocrine tumors there are many different tumor types:[14] this outline is presented to facilitate retrieving information. It is quite clear that neuroendocrine tumors are uncommon in many of these areas, and frequently represent only a very small proportion of the tumors or cancers at these locations.
- Pituitary
- neuroendocrine tumor of the anterior pituitary
- Thyroid tumors, particularly medullary carcinoma
- Parathyroid tumors
- Thymus and mediastinal carcinoid tumors[15][16]
- Pulmonary neuroendocrine tumors[17][18]
- bronchus[16]
- pulmonary carcinoid tumors: typical carcinoid (TC; low-grade); atypical carcinoid (AC; intermediate-grade)
- small-cell lung cancer (SCLC)
- large-cell neuroendocrine carcinoma (LCNEC of the lung)[19]
- Extrapulmonary small cell carcinomas (ESCC or EPSCC)
- Gastroenteropancreatic neuroendocrine tumors (GEP-NET)[20][21]
- Foregut GEP-NET (foregut tumors can conceptually encompasses not only NETs of the stomach and proximal duodenum, but also the pancreas, and even thymus, lung and bronchus)
{{citation}}: Empty citation (help)- Pancreatic endocrine tumors (if considered separately from foregut GEP-NET)[22]
- Midgut GEP-NET (from distal half of 2nd part of the duodenum to the proximal two-thirds of the transverse colon)
- appendix,[23] including well differentiated NETs (benign); well differentiated NETs (uncertain malignant potential); well differentiated neuroendocrine carcinoma (with low malignant potential); mixed exocrine-neuroendocrine carcinoma (goblet cell carcinoma, also called adenocarcinoid and mucous adenocarcinoid)
- Hindgut GEP-NET[24][25]
- Foregut GEP-NET (foregut tumors can conceptually encompasses not only NETs of the stomach and proximal duodenum, but also the pancreas, and even thymus, lung and bronchus)
- Liver[26][27][28] and gallbladder[29]
- Adrenal tumors, particularly adrenomedullary tumors
- Pheochromocytoma
- Peripheral nervous system tumors, such as:
- Breast[30]
- Genitourinary tract
- Merkel cell carcinoma of skin (trabecular cancer)
- Several inherited conditions:[34]
TNM stage
A TNM scheme has been proposed for NETs by the European Neuroendocrine Tumor Society.[10]
Histopathology
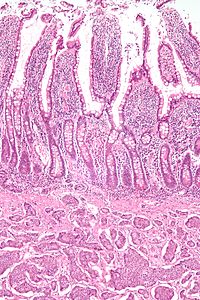
Features in common
Neuroendocrine tumors, despite differing embryological origin, have
common phenotypic characteristics. {{citation}}: Empty citation (help)
NETs show tissue immunoreactivity for markers of neuroendocrine differentiation (pan-neuroendocrine tissue markers) and may secrete various peptides and hormones. There is a lengthy list of potential markers in neuroendocrine tumors; several reviews provide assistance in understanding these markers.[40][41] Widely used neuroendocrine tissue markers are various chromogranins, synaptophysin and PGP9.5. Neuron-specific enolase (NSE) is less specific.[1][12]
NETs are often small, yellow or tan masses, often located in the submucosa or more deeply intramurally, and they can be very firm due to an accompanying intense desmoplastic reaction. The overlying mucosa may be either intact or ulcerated. Some GEP-NETs invade deeply to involve the mesentery.
Histologically, NETs are an example of "small blue cell tumors," showing uniform cells which have a round to oval stippled nucleus and scant, pink granular cytoplasm. The cells may align variously in islands, glands or sheets. High power examination shows bland cytopathology. Electron microscopy can identify secretory granules. There is usually minimal pleomorphism but less commonly there can be anaplasia, mitotic activity, and necrosis.
Some neuroendocrine tumor cells possess especially strong hormone receptors, such as somatostatin receptors and uptake hormones strongly. This avidity can assist in diagnosis and may make some tumors vulnerable to hormone targeted therapies.
Argentaffin and hormone secretion
NETs from a particular anatomical origin often show similar behavior as a group, such as the foregut (which conceptually includes pancreas, and even thymus, airway and lung NETs), midgut and hindgut; individual tumors within these sites can differ from these group benchmarks:
- Foregut NETs are argentaffin negative. Despite low serotonin content, they often secrete 5-hydroxytryptophan (5-HTP), histamine, and several polypeptide hormones. There may be associated atypical carcinoid syndrome, acromegaly, Cushing disease, other endocrine disorders, telangiectasia, or hypertrophy of the skin in the face and upper neck.[42] These tumors can metastasize to bone.
- Midgut NETs are argentaffin positive, can produce high levels of serotonin 5-hydroxytryptamine (5-HT), kinins, prostaglandins, substance P (SP), and other vasoactive peptides, and sometimes produce corticotropic hormone (previously adrenocorticotropic hormone [ACTH]). Bone metastasis is uncommon.
- Hindgut NETs are argentaffin negative and rarely secrete 5-HT, 5-HTP, or any other vasoactive peptides. Bone metastases are not uncommon.
Symptoms
GEP-NET (Gastroenteropancreatic Neuroendocrine Tumors)
There are two main types of NET within this category:
Carcinoid tumors
(about two thirds of GEP-NETs)
Carcinoids most commonly affect the small bowel, particularly the ileum, and are the most common malignancy of the appendix. Many carcinoids are asymptomatic and are discovered only upon surgery for unrelated causes. These coincidental carcinoids are common; one study found that one person in ten has them.[43] Many tumors do not cause symptoms even when they have metastasized.[4] Other tumors even if very small can produce adverse effects by secreting hormones.[44]
10%[45] or less of carcinoids, primarily some midgut carcinoids, secrete excessive levels of a range of hormones, most notably serotonin (5-HT) or substance P,[46] causing a constellation of symptoms called carcinoid syndrome:
- flushing
- diarrhea
- asthma or wheezing
- congestive heart failure (CHF)
- abdominal cramping
- peripheral edema
- heart palpitations
A carcinoid crisis with profound flushing, bronchospasm, tachycardia, and widely and rapidly fluctuating blood pressure[1] can occur if large amounts of hormone are acutely secreted,[46] which is occasionally triggered by factors such as diet,[46] alcohol,[46] surgery[1][46] chemotherapy,[46] embolization therapy[1] or radiofrequency ablation.[1]
Chronic exposure to high levels of serotonin causes thickening of the heart valves, particularly the tricuspid and the pulmonic valves, and over a long period can lead to congestive heart failure.[46] However, valve replacement is rarely needed.[47] The excessive outflow of serotonin can cause a depletion of tryptophan leading to niacin deficiency, and thus pellagra,[1] which is associated with dermatitis, dementia, and diarrhea.
Many other hormones can be secreted by some of these tumors, most commonly growth hormone that can cause acromegaly, or cortisol, that can cause Cushing's syndrome.
Occasionally, haemorrhage or the effects of tumor bulk are the presenting symptoms. Bowel obstruction can occur, sometimes due to fibrosing effects of NET secretory products[44] with an intense desmoplastic reaction at the tumor site, or of the mesentery.
Pancreatic endocrine tumors (PETs)
(about one third of GEP-NETs)
Pancreatic neuroendocrine tumors (PETs or PNETs; not to be confused with the primitive neuroectodermal PNET) can originate within the pancreas or from similar neuroendocrine cells outside of the pancreas. It is unclear whether pancreatic tumors originate from the usual cells of the islet of Langerhans or from diffuse neuroendocrine pluripotent cells.[4] PNETs are quite distinct from the usual form of pancreatic cancer, adenocarcinoma, which arises in the exocrine pancreas. About 95 percent of pancreatic tumors are adenocarcinoma; only 1 or 2% of clinically significant pancreas neoplasms are GEP-NETs.
Well or intermediately differentiated PNETs are sometimes called islet cell tumors; neuroendocrine cancer (NEC) is more aggressive.
About 70-85% PNETs are functional, secreting hormones that cause symptoms. About 15 to 30 percent of PETs are nonsecretory or nonfunctional, which either don’t secrete, or the quantity or type of products do not cause a clinical syndrome, such as pancreatic polypeptide (PPoma), chromogranin A, and neurotensin[34] although blood levels may be elevated. Functional tumors are often classified by the hormone most strongly secreted, for example:
- gastrinoma: the excessive gastrin causes Zollinger-Ellison Syndrome (ZES) with peptic ulcers and diarrhea
- insulinoma:[48] hypoglycemia occurs with concurrent elevations of insulin, proinsulin and C peptide[47]
- glucagonoma: the symptoms are not all due to glucagon elevations,[47] and include a rash, sore mouth, altered bowel habits, venous thrombosis, and high blood glucose levels[47]
- VIPoma, producing excessive vasoactive intestinal peptide, which may cause profound chronic watery diarrhea and resultant dehydration, hypokalemia, and achlorhydria (WDHA or pancreatic cholera syndrome)
- somatostatinoma: these rare tumors are associated with elevated blood glucose levels, achlorhydria, cholelithiasis, and diarrhea[47]
- less common types include ACTHoma, CRHoma, calcitoninoma, GHRHoma, GRFoma, and parathyroid hormone–related peptide tumor
In these functional tumors, the frequency of malignancy and the survival prognosis have been estimated dissimilarly, but a pertinent accessible summary is available.[1]
Other
In addition to the two main categories, there are rarer forms of GEP-NETs, including neuroendocrine lung, thymus and parathyroid tumors. Bronchial carcinoid can cause airway obstruction, pneumonia, pleurisy, difficulty with breathing, cough, and hemoptysis, or may be associated with weakness, nausea, weight loss, night sweats, neuralgia, and Cushing’s syndrome. Some are asymptomatic.
Animal neuroendocrine tumors include neuroendocrine cancer of the liver in dogs, and Devil facial tumor disease in Tasmanian Devils.[49][50][51]
Diagnosis
Markers
Symptoms from secreted hormones may prompt measurement of the corresponding hormones in the blood or their associated urinary products, for initial diagnosis or to assess the interval change in the tumor. Secretory activity of the tumor cells is sometimes dissimilar to the tissue immunoreactivity to particular hormones.[41]
Given the diverse secretory activity of NETs there are many other potential markers, but a limited panel is usually sufficient for clinical purposes.[1] Aside from the hormones of secretory tumors, the most important markers are :
- chromogranin A (CgA) [citation needed]
- urine 5-hydroxyindoleacetic acid (5-HIAA)
- neuron-specific enolase (NSE, gamma-gamma dimer)
- synaptophysin (P38)
Newer markers include N-terminally truncated variant of Hsp70 is present in NETs but absent in normal pancreatic islets.[52] High levels of CDX2, a homeobox gene product essential for intestinal development and differentiation, are seen in intestinal NETs.[52] Neuroendocrine secretory protein-55, a member of the chromogranin family, is seen in pancreatic endocrine tumors but not intestinal NETs.[52]
Imaging
CT-scans, MRIs, sonography (ultrasound), and endoscopy (including endoscopic ultrasound) are common diagnostic tools. CT-scans using contrast medium can detect 95 percent of tumors over 3 cm in size, but generally not tumors under 1 cm.[10]
Advances in nuclear medicine imaging, also known as molecular imaging, has improved diagnostic and treatment paradigms in patients with neuroendocrine tumors. This is because of its ability to not only identify sites of disease but also characterize them. Neuronedocrine tumours express somatostatin receptors providing a unique target for imaging. Octreotide is a synthetic modifications of somatostatin with a longer half-life. OctreoScan, also called somatostatin receptor scintigraphy (SRS or SSRS), utilizes intravenously administered octreotide that is chemically bound to a radioactive substance, often indium-111, to detect larger lesions with tumor cells that are avid for octreotide.
Somatostatin receptor imaging can now be performed with positron emission tomography (PET) which offers higher resolution, three-dimensional and more rapid imaging. Gallium-68 receptor PET-CT is much more accurate than an OctreoScan.[53]
Imaging with fluorine-18 fluorodeoxyglucose (FDG) PET is also valuable to image neuroendocrine tumors.[54] This scan is performed by injected radioactive sugar intravenously. Tumors that grow more quickly use more sugar. Using this scan, the aggressiveness of the tumor can be visualised.
The combination of somatostatin receptor and FDG PET imaging is able to quantify somatostatin receptor cell surface (SSTR) expression and glycolytic metabolism, respectively.[54] The ability to perform this as a whole body study is highlighting the limitations of relying on histopathology obtained from a single site. This is enabling better selection of the most appropriate therapy for an individual patient.
Genetics
Pancreatic neuroendocrine tumors
DNA mutation analysis in well-differentiated pancreatic neuroendocrine tumors identified four important findings:[55]
- as expected, the genes mutated in NETs, MEN1, ATRX, DAXX, TSC2, PTEN and PIK3CA,[55] are different from the mutated genes previously found in pancreatic adenocarcinoma.[56][57]
- one in six well-differentiated pancreatic NETs have mutations in mTOR pathway genes, such as TSC2, PTEN and PIK3CA.[55] The sequencing discovery might allow selection of which NETs would benefit from mTOR inhibition such as with everolimus, but this awaits validation in a clinical trial.
- mutations affecting a new cancer pathway involving ATRX and DAXX genes were found in about 40% of pancreatic NETs.[55] The proteins encoded by ATRX and DAXX participate in chromatin remodeling of telomeres;[58] these mutations are associated with a telomerase-independent maintenance mechanism termed ALT (alternative lengthening of telomeres) that results in abnormally long telomeric ends of chromosomes.[58]
Familial syndromes
Neuroendocrine tumors can be seen in several inherited familial syndromes,[34] including:
- multiple endocrine neoplasia type 1 (MEN1)
- multiple endocrine neoplasia type 2 (MEN2)
- von Hippel-Lindau (VHL) disease[34]
- neurofibromatosis type 1[35]
- tuberous sclerosis[36][37]
- Carney complex[38][39]
Recommendations in NET include family history evaluation, evaluation for second tumors, and in selected circumstances testing for germline mutations such as for MEN1.[1]
Treatment
Overview
Several issues help define appropriate treatment of a neuroendocrine tumor, including its location, invasiveness, hormone secretion, and metastasis. Treatments may be aimed at curing the disease or at relieving symptoms (palliation).
Observation may be feasible for non-functioning low grade neuroendocrine tumors.[citation needed]
If the tumor is locally advanced or has metastasized, but is nonetheless slowly growing, treatment that relieves symptoms may often be preferred over immediate challenging surgeries.[citation needed]
Intermediate and high grade tumors (noncarcinoids) are usually best treated by various early interventions (active therapy) rather than observation (wait-and-see approach).[5]
Treatments have improved over the past several decades, and outcomes are improving.[44] In malignant carcinoid tumors with carcinoid syndrome, the median survival has improved from two years to more than eight years.[6]
Detailed guidelines for managing neuroendocrine tumors are available from ESMO,[59] NCCN[60] and a UK panel.[1] The NCI has guidelines for several categories of NET: islet cell tumors of the pancreas,[61] gastrointestinal carcinoids,[62] Merkel cell tumors[63] and pheochromocytoma and paraganglioma.[64]
Surgery
Surgery is a curative treatment for some neuroendocrine tumors.
Even if the tumor has advanced and metastasized, making curative surgery infeasible, surgery often has a role in neuroendocrine cancers for palliation of symptoms and possibly improved survival.[5]
Symptomatic relief
In secretory tumors, somatostatin analogs given subcutaneously or intramuscularly alleviate symptoms by blocking hormone release. A consensus review has reported on the use of somatostatin analogs for GEP-NETs.[65]
These medications may also anatomically stabilize or shrink tumors, as suggested by the PROMID study (Placebo-controlled prospective randomized study on the antiproliferative efficacy of Octreotide LAR in patients with metastatic neuroendocrine MIDgut tumors): at least in this subset of NETs, average tumor stabilization was 14.3 months compared to 6 months for placebo.[66]
Other medications that block particular secretory effects can sometimes relieve symptoms.[47]
Chemotherapy
Interferon is sometimes used to treat GEP-NETs.[67] Its effectiveness is somewhat uncertain, but low doses can be titrated within each person, often considering the effect on the blood leukocyte count;[67] interferon is often used in combination with other agents, especially somatostatin analogs such as octreotide.
Gastrointestinal neuroendocrine tumors
Most gastrointestinal carcinoid tumors tend not to respond to chemotherapy agents,[47] showing 10 to 20% response rates that are typically less than 6 months.[47] Combining chemotherapy medications has not usually been of significant improvement[47] showing 25 to 35% response rates that are typically less than 9 months. The exceptions are poorly-differentiated (high-grade or anaplastic) metastatic disease, where cisplatin with etoposide may be used[47] and Somatostatin Receptor Scintigraphy (SSRS) negative tumors which had a response rate in excess of 70% compared to 10% in strongly positive SRSS carcinoid tumors.[1]
Pancreatic endocrine tumors
PETs are more responsive to chemotherapy than are gastroenteric carcinoid tumors. Several agents have shown activity[47] and combining several medicines, particularly doxorubicin with streptozocin, is often more effective.[47] Although marginally effective in well-differentiated PETs, cisplatin with etoposide is active in poorly-differentiated neuroendocrine cancers (PDNECs).[47]
Targeted chemotherapy agents have been approved in PETs by the FDA based on improved progression-free survival (PFS):
- everolimus (Afinitor) is labeled for treatment of progressive neuroendocrine tumors of pancreatic origin in patients with unresectable, locally advanced or metastatic disease.[68][69] The safety and effectiveness of everolimus in carcinoid tumors have not been established.[68][69]
- sunitinib (Sutent) is labeled for treatment of progressive, well-differentiated pancreatic neuroendocrine tumors (pNET) in patients with unresectable locally advanced or metastatic disease.[70][71] Sutent also has approval from the European Commission for the treatment of 'unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumors with disease progression in adults'.[72] A phase III study of sunitinib treatment in patients with advanced and well differentiated pNET showed that sunitinib treatment improved progression-free survival (11.4 months vs. 5.5 months), overall survival, and the objective response rate (9.3% vs. 0.0%) when compared with placebo.[73]
Peptide Receptor Radionuclide Therapy (PRRT)
In this type of radioisotope therapy (RIT)[13] the tumor is treated intravenously with a peptide or hormone conjugated to a radionuclide or radioligand, the peptide or neuroamine hormone previously having shown good uptake of a tracer dose. This kind of RIT may be called peptide receptor radionuclide therapy (PRRT), or hormone-delivered radiotherapy, and can attack all lesions in the body, not just liver metastases. This is typically by radiolabeling octreotate to lutetium-177, yttrium-90 or indium-111. This is a highly targeted and effective therapy with minimal side effects in tumours with high levels of somatostatin cell surface expression. This is because the radiation is absorbed at the sites of the tumour, or excreted in the urine. The radioactively labelled hormones enter the tumor cells which, together with nearby cells, are damaged by the attached radiation. Not all cells are immediately killed; cell death can go on for up to two years. Early referral to a nuclear medicine physician is suggested to assess suitability for this new treatment modality. Somatostatin receptor imaging as detailed above can help determine suitability for PRRT.
Hepatic artery
Metastases to the liver can be treated by several types of hepatic artery treatments based on the observation that tumor cells get nearly all their nutrients from the hepatic artery, while the normal cells of the liver get about 70-80 percent of their nutrients and 50% their oxygen supply from the portal vein, and thus can survive with the hepatic artery effectively blocked. [44][74]
Hepatic artery embolization (HAE) occludes the blood flow to the tumors,[46] achieving significant tumor shrinkage in over 80%.[46]
In hepatic artery chemotherapy, the chemotherapy agents are given into the hepatic artery, often by steady infusion over hours or even days. Compared with systemic chemotherapy, a higher proportion of the chemotherapy agents are (in theory) delivered to the lesions in the liver. [74]
Hepatic artery chemoembolization (HACE), sometimes called transarterial chemoembolization (TACE), combines hepatic artery embolization with hepatic artery chemoinfusion: embospheres bound with chemotherapy agents, injected into the hepatic artery, lodge in downstream capillaries. The spheres not only block blood flow to the lesions, but by halting the chemotherapy agents in the neighborhood of the lesions, they provide a much better targeting leverage than chemoinfusion provides.
Selective internal radiation therapy (SIRT)[75] for neuroendocrine metastases to the liver[76] delivers radioactive microsphere therapy (RMT) by injection into the hepatic artery, lodging (as with HAE and HACE) in downstream capillaries. In contrast to hormone-delivered radiotherapy, the lesions need not overexpress peptide receptors. The mechanical targeting delivers the radiation from the yttrium-labeled microspheres selectively to the tumors without unduly affecting the normal liver.[77] This type of treatment is FDA approved for liver metastases secondary to colorectal carcinoma and is under investigation for treatment of other liver malignancies, including neuroendocrine malignancies.[75]
Other therapies
Radiofrequency ablation (RFA) is used when a patient has relatively few metastases.[citation needed] In RFA, a needle is inserted into the center of the lesion and current is applied to generate heat; the tumor cells are killed by cooking.
Cryoablation is similar to RFA; an endothermic substance[citation needed] is injected into the tumors to kill by freezing. Cryoablation has been considerably less successful for GEP-NETs than RFA.[citation needed]
References
- ^ a b c d e f g h i j k l m n o Ramage JK, Davies AH, Ardill J; et al. (2005). "Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours". Gut. 54. Suppl 4 (suppl_4): iv1–16. doi:10.1136/gut.2004.053314. PMC 1867801. PMID 15888809.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1111/j.1749-6632.1994.tb17251.x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1111/j.1749-6632.1994.tb17251.xinstead. - ^ a b c d e f Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.humpath.2004.09.018, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.humpath.2004.09.018instead. - ^ a b c d e Arnold R, Göke R, Wied M, Behr T (2003). "Chapter 15 Neuroendocrine Gastro-Entero-Pancreatic (GEP) Tumors". In Scheppach W, Bresalier RS, Tytgat GNJ (ed.). Gastrointestinal and Liver Tumors. Berlin: Springer. pp. 195–233. ISBN 3-540-43462-3.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1053/j.gastro.2005.03.078, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1053/j.gastro.2005.03.078instead. - ^ a b c Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10388123, please use {{cite journal}} with
|pmid=10388123instead. - ^ a b c d e f Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1097/MPA.0b013e3181ec124e, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1097/MPA.0b013e3181ec124einstead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/s10555-011-9292-1, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/s10555-011-9292-1instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF01297144, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF01297144instead. - ^ a b c d Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.5306/wjco.v2.i1.28, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.5306/wjco.v2.i1.28instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1111/j.1365-2559.2007.02883.x, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1111/j.1365-2559.2007.02883.xinstead. - ^ a b Liu Y, Sturgis CD, Grzybicki DM; et al. (2001). "Microtubule-associated protein-2: a new sensitive and specific marker for pulmonary carcinoid tumor and small cell carcinoma". Mod Pathol. 14 (9): 880–5. doi:10.1038/modpathol.3880406. PMID 11557784.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Rufini V, Calcagni ML, Baum RP (2006). "Imaging of neuroendocrine tumors". Semin Nucl Med. 36 (3): 228–47. doi:10.1053/j.semnuclmed.2006.03.007. PMID 16762613.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15053292, please use {{cite journal}} with
|pmid=15053292instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 10550713, please use {{cite journal}} with
|pmid=10550713instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdn116, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdn116instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 15898407, please use {{cite journal}} with
|pmid=15898407instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/cncr.23542, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/cncr.23542instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 1317668, please use {{cite journal}} with
|pmid=1317668instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.3748/wjg.14.5377, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.3748/wjg.14.5377instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID
18177818, please use {{cite journal}} with
|pmid= 18177818instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1053/j.gastro.2008.05.047, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1053/j.gastro.2008.05.047instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21160597, please use {{cite journal}} with
|pmid=21160597instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 20380002, please use {{cite journal}} with
|pmid=20380002instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21160865, please use {{cite journal}} with
|pmid=21160865instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12636090, please use {{cite journal}} with
|pmid=12636090instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1080/13651820310017228, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1080/13651820310017228instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 8348490, please use {{cite journal}} with
|pmid=8348490instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12725316, please use {{cite journal}} with
|pmid=12725316instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11890336, please use {{cite journal}} with
|pmid=11890336instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 17076534, please use {{cite journal}} with
|pmid=17076534instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 7511278, please use {{cite journal}} with
|pmid=7511278instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 11718210, please use {{cite journal}} with
|pmid=11718210instead. - ^ a b c d e Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/cncr.23648, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/cncr.23648instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/bja/86.4.555, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/bja/86.4.555instead. - ^ a b c Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.beem.2010.02.002, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.beem.2010.02.002instead. - ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1677/ERC-08-0142, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1677/ERC-08-0142instead. - ^ a b OMIM - Online Mendelian Inheritance in Man. Carney Complex, type 1; CNC1 (OMIM 160980)http://www.omim.org/entry/160980
- ^ a b OMIM - Online Mendelian Inheritance in Man. Carney Complex, type 2; CNC2 (OMIM 605244)
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.2741/s68, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.2741/s68instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18401212, please use {{cite journal}} with
|pmid=18401212instead. - ^ Tebbi CK. Carcinoid Tumor. http://emedicine.medscape.com/article/986050-overview
- ^ Kimura W, Kuroda A, Morioka Y (1991). "Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases". Dig Dis Sci. 36 (7): 933–42. doi:10.1007/BF01297144. PMID 2070707.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)"[In] 800 autopsy cases, ... incidence of tumor was 10% (6/60) in individuals having histological studies of all sections of the pancreas" - ^ a b c d Pommier R. 2003. The role of surgery and chemoembolization in the management of carcinoid. California Carcinoid Fighters Conference. October 25, http://www.carcinoid.org/content/role-surgery-and-chemoembolization-management-carcinoid
- ^ Health Communities. Carcinoid Tumor Overview. http://www.healthcommunities.com/carcinoid-malignancy/carcinoid-malignancy-overview.shtml
- ^ a b c d e f g h i Kvols LK. 2002. Carcinoid Tumors and the Carcinoid Syndrome: What's New in the Therapeutic Pipeline. (The Carcinoid Cancer Foundation: Carcinoid Symposium 2002)http://www.carcinoid.org/content/carcinoid-tumors-and-carcinoid-syndrome-whats-new-therapeutic-pipeline
- ^ a b c d e f g h i j k l m Benson AB, Myerson RJ, and Sasson AR. Pancreatic, neuroendocrine GI, and adrenal cancers. Cancer Management: A Multidisciplinary Approach 13th edition 2010. ISBN 978-0-615-41824-7 Text is available electronically (but may require free registration) at http://www.cancernetwork.com/cancer-management/pancreatic/article/10165/1802606
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16253900, please use {{cite journal}} with
|pmid=16253900instead. - ^ Bostanci A. Wildlife biology. A devil of a disease. Science 2005; 307:1035 pmid=15718445 doi=10.1126/science.307.5712.1035 "The tumors [of Devil facial tumor disease] have been characterized as a neuroendocrine cancer"
- ^ Kinver, Mark (1 January 2010). "Tasmanian devil facial cancer origins 'identified'". BBC.
- ^ Walsh, Bryan (1 January 2010). "Decoding the Tasmanian Devil's Deadly Cancer". Time.
- ^ a b c Oberg K (2005). "Neuroendocrine tumors of the gastrointestinal tract: recent advances in molecular genetics, diagnosis, and treatment". Curr Opin Oncol. 17 (4): 386–91. doi:10.1097/01.cco.0000167739.56948.a9. PMID 15933475.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 22339744, please use {{cite journal}} with
|pmid=22339744instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 22846204, please use {{cite journal}} with
|pmid=22846204instead. - ^ a b c d e Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1126/science.1200609, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1126/science.1200609instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 18772397, please use {{cite journal}} with
|pmid=18772397instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19077451, please use {{cite journal}} with
|pmid=19077451instead. - ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 21719641, please use {{cite journal}} with
|pmid=21719641instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdq192, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdq192instead. - ^ http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- ^ National Cancer Institute. Islet Cell Tumors (Endocrine Pancreas) http://www.cancer.gov/cancertopics/types/isletcell
- ^ National Cancer Institute. Gastrointestinal Carcinoid Tumors Treatmenthttp://www.cancer.gov/cancertopics/pdq/treatment/gastrointestinalcarcinoid/HealthProfessional
- ^ National Cancer Institute. Merkel cell tumors http://www.cancer.gov/cancertopics/pdq/treatment/merkelcell/Patient
- ^ National Cancer Institute. Pheochromocytoma and Paragangliomahttp://www.cancer.gov/cancertopics/types/pheochromocytoma
- ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1093/annonc/mdh216, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1093/annonc/mdh216instead. - ^ http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=63&abstractID=10363
- ^ a b Öberg K. Neuroendocrine Gastroenteropancreatic Tumours: Current Views on Diagnosis and Treatment. Business Briefing. European Oncology Review 2005; pages 1-6. http://www.touchbriefings.com/pdf/1432/ACF237.pdf
- ^ a b Everolimus Approved for Pancreatic Neuroendocrine Tumors. The ASCO Post. May 15, 2011, Volume 2, Issue 8 http://ascopost.com/articles/may-15-2011/everolimus-approved-for-pancreatic-neuroendocrine-tumors/
- ^ a b http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf
- ^ National Cancer Institute. Cancer Drug Information. FDA Approval for Sunitinib Malate. Pancreatic Neuroendocrine Tumors http://www.cancer.gov/cancertopics/druginfo/fda-sunitinib-malate
- ^ http://labeling.pfizer.com/ShowLabeling.aspx?id=607
- ^ "Pfizer Scores New Approval for Sutent in Europe". 2 Dec 2010.
- ^ Raymond E., Dahan L., Raoul J. L., Bang Y. J., Borbath I., Lombard-Bohas C., Valle J., Metrakos P.; et al. (2011). "Sunitinib malate for the treatment of pancreatic neuroendocrine tumors". N Engl J Med. 364 (6): 501–13. PMID 21306237.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ a b Fong, T and Schoenfield LJ. Arterial Chemotherapy Infusion of the Liver (and) Chemoembolization of the Liver (TACE)[1]).
- ^ a b Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 16979443, please use {{cite journal}} with
|pmid=16979443instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 19521311, please use {{cite journal}} with
|pmid=19521311instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12354840, please use {{cite journal}} with
|pmid=12354840instead.
External links
- http://www.carcinoid.org
- Johns Hopkins University School of Medicine Pancreatic Neuroendocrine Tumor Page
- http://unicornfoundation.org.au
- Neuroendocrine tumor at Curlie
- The Caring for Carcinoid Foundation
Template:Gonadal tumors, paraganglioma, glomus, nevi and melanomas
