Iclaprim
 | |
| Clinical data | |
|---|---|
| Routes of administration | intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.130.860 |
| Chemical and physical data | |
| Formula | C19H22N4O3 |
| Molar mass | 354.410 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Iclaprim is an antibiotic drug candidate that is active against Gram positive organisms.[1][2] It is administered intravenously.[3]: 3
In vitro, iclaprim is active against methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Staphylococcus aureus (VRSA), strains of Streptococcus pneumoniae resistant to several common antibiotics, and some Gram-negative bacteria.[4] It is of the diaminopyrimidine dihydrofolate reductase (DHFR)-inhibiting type.
History
Iclaprim is an optimized analog of trimethoprim that was discovered by scientists at Roche.[5] Arpida was spun out of Roche in 1998,[5][6] and acquired iclaprim from Roche in 2001.[7]: 77 Arpida held an initial public offering on the Swiss stock exchange in 2005.[8]
Arpida ran two Phase III clinical trials for complicated skin and skin structure infections that were completed by 2008, but as of 2017, had not been published in the medical literature.[2] A new drug application was filed with the United States Food and Drug Administration based on these trials, and was rejected due to failure to show non-inferiority and due to safety concerns, especially drug-induced QT prolongation.[2][9] The FDA advisory committee said that the drug "should not be developed further" based on the results presented.[10] A parallel application for marketing approval to the European Medicines Agency was withdrawn in 2009; in the announcement of the withdrawal, the EMA said that there was insufficient data from clinical studies to justify the dosage proposed by the company and that resistance to the drug had already been seen in the clinical trial data.[11]
Arpida collapsed after the rejection by the FDA and the EMA withdrawal.[6] Arpida and the privately owned Swiss company Evolva began discussing an acquisition of Arpida by Evolva, which would allow Evolva to go public via a reverse merger in September 2009.[12] Arpida sold off iclaprim to Acino Pharma in November 2009,[13] and in December 2009, Arpida and Evolva completed their transaction.[14]
Acino sold the rights to iclaprim, its data and regulatory filings, and manufactured drug to a group called Life Sciences Management Group of Bethesda, Maryland, in September 2013 and that company assigned its rights to a company called Nuprim,[7]: F-32 which had been formed by the former chief executive officer, chief science officer, and US agent of Arpida in 2014.[15] In December 2014, Motif BioSciences and Nuprim signed an agreement allowing Motif to acquire the iclaprim assets, and the transaction was completed in April 2015.[7]: iv In 2015, the FDA granted qualified infectious disease product status for iclaprim.[10]
In September 2017, the FDA granted orphan drug status to iclaprim for the treatment of Staphylococcus aureus lung infections in people with cystic fibrosis.[16] Iclaprim was non-inferior to vancomycin when it was studied in two phase III studies of acute skin and skin structure infections published in 2018.[17][18] As of February 2019, it is still not approved.
Chemistry
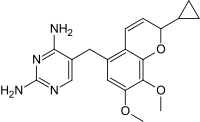
Iclaprim contains a stereocenter and is a racemate, a 1: 1 mixture of (R)- and (S)-enantiomers:
| Enantiomers of iclaprim | |
|---|---|
 CAS number: 1208116-65-7 |
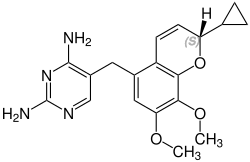 CAS number: 1208116-66-8 |
Names
During its development, other names for the drug have included AR-100, MTF-100, RO-48-2622,[19] and the brand name Mersarex.[11] It received its INN name in 2003.[20]
References
- ^ Barton E, MacGowan A (December 2009). "Future treatment options for Gram-positive infections--looking ahead". Clinical Microbiology and Infection. 15 (Suppl 6): 17–25. doi:10.1111/j.1469-0691.2009.03055.x. PMID 19917023.
- ^ a b c Abbas M, Paul M, Huttner A (October 2017). "New and improved? A review of novel antibiotics for Gram-positive bacteria". Clinical Microbiology and Infection. 23 (10): 697–703. doi:10.1016/j.cmi.2017.06.010. PMID 28642145.
- ^ "Iclaprim for the Treatment of Complicated Skin and Skin Structure Infections" (PDF). FDA. November 20, 2008.
- ^ Kohlhoff SA, Sharma R (September 2007). "Iclaprim". Expert Opinion on Investigational Drugs. 16 (9): 1441–1448. doi:10.1517/13543784.16.9.1441. PMID 17714029. S2CID 219289697.
- ^ a b Firn D (11 October 2004). "Arpida takes advice on stock market flotation". Financial Times.
- ^ a b Silver LL (January 2011). "Challenges of antibacterial discovery". Clinical Microbiology Reviews. 24 (1): 71–109. doi:10.1128/CMR.00030-10. PMC 3021209. PMID 21233508.
- ^ a b c "Form F-1". Motif Bio plc via SEC Edgar. July 12, 2016.
- ^ Thompson V (April 12, 2005). "Roche spin-off plans float to fund drug trials". SWI swissinfo.ch.
- ^ "Summary Minutes of the Anti-Infective Drugs Advisory Committee November 18-20, 2008" (PDF). US Food and Drug Administration.. "Iclaprim for the Treatment of Complicated Skin and Skin Structure Infections FDA Briefing Document for Anti-Infective Drugs Advisory Committee Meeting" (PDF). US Food and Drug Administration. 20 November 2008.
- ^ a b King A (3 August 2015). "FDA veteran questions science behind antibiotics fast track". In-PharmaTechnologist.
- ^ a b "Questions and answers on the withdrawal of the marketing authorisation application for Mersarex (iclaprim)" (PDF). EMA. 19 November 2009.
- ^ "Privately owned Evolva takes over biotech Arpida". Reuters. September 10, 2009.
- ^ "Press Release: Acino Acquires Iclaprim Activities from Arpida". Acino, Roche, Arpida via Evaluate Group. November 4, 2009.
- ^ "Press Release: Evolva SA Completes Merger With Arpida Ltd". Evolva Holding, Novartis, Arpida via Evaluate Group. December 14, 2009.
- ^ "Press release: Motif Bio Announces Intention to Float on AIM". Motif Bio plc. February 2, 2015. Archived from the original on 17 March 2016.
- ^ "Iclaprim Orphan Drug Designation". FDA. Retrieved 16 September 2017.
- ^ Huang DB, O'Riordan W, Overcash JS, Heller B, Amin F, File TM, et al. (April 2018). "A Phase 3, Randomized, Double-Blind, Multicenter Study to Evaluate the Safety and Efficacy of Intravenous Iclaprim Vs Vancomycin for the Treatment of Acute Bacterial Skin and Skin Structure Infections Suspected or Confirmed to be Due to Gram-Positive Pathogens: REVIVE-1". Clinical Infectious Diseases. 66 (8): 1222–1229. doi:10.1093/cid/cix987. PMID 29281036.
- ^ Holland TL, O'Riordan W, McManus A, Shin E, Borghei A, File TM, et al. (May 2018). "A Phase 3, Randomized, Double-Blind, Multicenter Study To Evaluate the Safety and Efficacy of Intravenous Iclaprim versus Vancomycin for Treatment of Acute Bacterial Skin and Skin Structure Infections Suspected or Confirmed To Be Due to Gram-Positive Pathogens (REVIVE-2 Study)". Antimicrobial Agents and Chemotherapy. 62 (5): e02580-17. doi:10.1128/AAC.02580-17. PMC 5923167. PMID 29530858.
- ^ "Iclaprim". AdisInsight. Springer Nature Switzerland AG. Retrieved 13 September 2017.
- ^ "Recommended INN List 50" (PDF). WHO Drug Information. 17 (4). 2003.
External links
Further reading
- Schneider P, Hawser S, Islam K (December 2003). "Iclaprim, a novel diaminopyrimidine with potent activity on trimethoprim sensitive and resistant bacteria". Bioorganic & Medicinal Chemistry Letters. 13 (23): 4217–4221. doi:10.1016/j.bmcl.2003.07.023. PMID 14623005.
