Anthranilic acid
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Aminobenzoic acid[1] | |||
| Systematic IUPAC name
2-Aminobenzenecarboxylic acid | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 471803 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.898 | ||
| EC Number |
| ||
| 3397 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H7NO2 | |||
| Molar mass | 137.138 g·mol−1 | ||
| Appearance | white or yellow solid | ||
| Odor | odorless | ||
| Density | 1.412 g/cm3 | ||
| Melting point | 146 to 148 °C (295 to 298 °F; 419 to 421 K)[3] | ||
| Boiling point | 200 °C (392 °F; 473 K) (sublimes) | ||
| 0.572 g/100 mL (25 °C) | |||
| Solubility | very soluble in chloroform, pyridine soluble in ethanol, ether, ethyl ether slightly soluble in trifluoroacetic acid, benzene | ||
| log P | 1.21 | ||
| Vapor pressure | 0.1 Pa (52.6 °C) | ||
| Acidity (pKa) |
| ||
| -77.18·10−6 cm3/mol | |||
Refractive index (nD)
|
1.578 (144 °C) | ||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-380.4 KJ/mol | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H318, H319 | |||
| P264, P280, P305+P351+P338, P310, P337+P313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | > 150 °C (302 °F; 423 K) | ||
| > 530 °C (986 °F; 803 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1400 mg/kg (oral, rat) | ||
| Safety data sheet (SDS) | External MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Anthranilic acid is an aromatic acid with the formula C6H4(NH2)(CO2H) and has a sweetish taste.[4][5][6] The molecule consists of a benzene ring, ortho-substituted with a carboxylic acid and an amine. As a result of containing both acidic and basic functional groups, the compound is amphoteric. Anthranilic acid is a white solid when pure, although commercial samples may appear yellow. The anion [C6H4(NH2)(CO2)]−, obtained by the deprotonation of anthranilic acid, is called anthranilate. Anthranilic acid was once thought to be a vitamin and was referred to as vitamin L1 in that context, but it is now known to be non-essential in human nutrition.[7]
Structure
Although not usually referred to as such, it is an amino acid. Solid anthranilic acid consists of both the amino-carboxylic acid and the zwitterionic ammonium carboxylate forms.[8]
Production
Many routes to anthranilic acid have been described. Industrially it is produced from phthalic anhydride, beginning with amination:
- C6H4(CO)2O + NH3 + NaOH → C6H4(C(O)NH2)CO2Na + H2O
The resulting sodium salt of phthalamic acid is decarbonylated via a Hofmann rearrangement of the amide group, induced by hypochlorite:[9]
- C6H4(C(O)NH2)CO2Na + HOCl → C6H4NH2CO2H + NaCl + CO2
A related method involves treating phthalimide with sodium hypobromite in aqueous sodium hydroxide, followed by neutralization.[10] In the era when indigo dye was obtained from plants, it was degraded to give anthranilic acid.
Anthranilic acid was first obtained by base-induced degradation of indigo.[11]
Biosynthesis
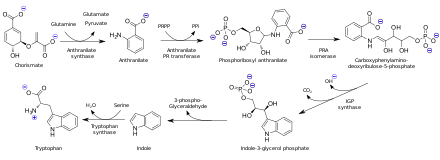
Anthranilic acid is biosynthesized from chorismic acid. In organisms capable of tryptophan synthesis, anthranilate is a precursor to the amino acid tryptophan via the attachment of phosphoribosyl pyrophosphate to the amine group.
Uses
Industrially, anthranilic acid is an intermediate in the production of azo dyes and saccharin. It and its esters are used in preparing perfumes to mimic jasmine and orange, pharmaceuticals (loop diuretics, such as furosemide) and UV-absorber as well as corrosion inhibitors for metals and mold inhibitors in soy sauce.
Anthranilate-based insect repellents have been proposed as replacements for DEET.
Fenamic acid is a derivative of anthranilic acid,[12]: 235 which in turn is a nitrogen isostere of salicylic acid, which is the active metabolite of aspirin.[12]: 235 Several non-steroidal anti-inflammatory drugs, including mefenamic acid, tolfenamic acid, flufenamic acid, and meclofenamic acid are derived from fenamic acid or anthranilic acid and are called "anthranilic acid derivatives" or "fenamates".[13]: 17
Reactions
Anthranilic acid can be diazotized to give the diazonium cation [C6H4(CO2H)(N2)]+. This cation can be used to generate benzyne,[14] dimerized to give diphenic acid,[15] or undergo diazonium coupling reactions such as in the synthesis of methyl red.[16]
It reacts with phosgene to give isatoic anhydride, a versatile reagent.[17]
Chlorination of anthranilic acid gives the 2,4-dichloro derivative, which can undergo reductive coupling to form a biaryl compound.[18]
Safety and regulation
It is also a DEA List I Chemical because of its use in making the now-widely outlawed euphoric sedative drug methaqualone (Quaalude, Mandrax).[19]
See also
References
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 748. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. pp. 5–89. ISBN 978-1498754286.
- ^ IPCS
- ^ Acton, Q. Ashton (2013). Aminobenzoic Acids—Advances in Research and Application (2013 ed.). Atlanta: ScholarlyEditions. p. 23. ISBN 9781481684842 – via Google Books.
- ^ Hardy, Mark R. (1997). "Glycan Labeling with the Flurophores 2-Aminobenzamide and Antranilic Acid". In Townsend, R. Reid; Hotchkiss, Jr., Arland T. (eds.). Techniques in Glycobiology. Marcel Dekker, Inc. p. 360. ISBN 9780824798222 – via Google Books.
- ^ The Merck Index, 10th Ed. (1983), p.62., Rahway: Merck & Co.
- ^ Davidson, Michael W. (2004). "Anthranilic Acid (Vitamin L)]". Florida State University. Retrieved November 20, 2019.
- ^ Brown, C. J. (1968). "The crystal structure of anthranilic acid". Proceedings of the Royal Society of London. Series A. Mathematical and Physical Sciences. 302 (1469): 185–199. Bibcode:1968RSPSA.302..185B. doi:10.1098/rspa.1968.0003.
- ^ Maki, Takao; Takeda, Kazuo (2000). "Benzoic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_555. ISBN 3527306730..
- ^ Vogel's Textbook of Practical Organic Chemistry, 4th Ed., (B. S. Furniss et al., Eds.) (1978), p.666, London: Longman.
- ^ Sheibley, Fred E. (1943). "Carl Julius Fritzsche and the discovery of anthranilic acid, 1841". Journal of Chemical Education. 20 (3): 115. Bibcode:1943JChEd..20..115S. doi:10.1021/ed020p115.
- ^ a b Sriram D, Yogeeswari P. Medicinal Chemistry, 2nd Edition. Pearson Education India, 2010. ISBN 9788131731444
- ^ Auburn University course material. Jack DeRuiter, Principles of Drug Action 2, Fall 2002 1: Non-Steroidal Antiinflammatory Drugs (NSAIDS)
- ^ Logullo, F. M.; Seitz, A. H.; Friedman, L. (1968). "Benzenediazonium-2-carboxy- and Biphenylene". Organic Syntheses. 48: 12.
- ^ Atkinson, E. R.; Lawler, H. J. (1927). "Diphenic Acid". Organic Syntheses. 7: 30. doi:10.15227/orgsyn.007.0030.
- ^ Clarke, H. T.; Kirner, W. R. (1922). "Methyl Red". Organic Syntheses. 2: 47.
- ^ Wagner, E. C.; Fegley, Marion F. (1947). "Isatoic anhydride". Org. Synth. 27: 45. doi:10.15227/orgsyn.027.0045.
- ^ Atkinson, Edward R.; Murphy, Donald M.; Lufkin, James E. (1951). "dl-4,4',6,6'-Tetrachlorodiphenic Acid". Organic Syntheses. 31: 96.
- ^ Angelos SA, Meyers JA (1985). "The isolation and identification of precursors and reaction products in the clandestine manufacture of methaqualone and mecloqualone". Journal of Forensic Sciences. 30 (4): 1022–1047. PMID 3840834.