Dalcetrapib
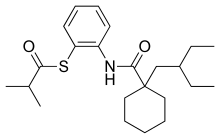
| |
| Names | |
|---|---|
| IUPAC name
S-[2-({[1-(2-Ethylbutyl)cyclohexyl]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.250.741 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H35NO2S | |
| Molar mass | 389.5945 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dalcetrapib (INN,[1] codenamed JTT-705) is a CETP inhibitor which was being developed by Hoffmann–La Roche until May 2012.[2][3] The drug was aimed at raising the blood levels of "good cholesterol" (cholesterol carried in HDL particles, aka HDL-C).[4] Prevailing observations indicate that high HDL levels correlate with better overall cardiovascular health, though it remains unclear whether raising HDL levels consequently leads to an increase in cardiovascular health.[5]
A 24-week clinical trial showed that dalcetrapib did increase HDL-C levels, supporting the agent's desired effect.[6] Further, the dal-PLAQUE phase IIb trial found evidence of plaque reduction.[7] Plaque reduction is an anticipated observation following an increase in HDL.[citation needed]
As of 2010[update] five phase II trials had started and there was no evidence of the raised blood pressure seen with torcetrapib.[6]
dal-VESSEL phase IIb trial found no evidence of flow-mediated dilatation improvement. A 17% increase of Lp-PLA2 mass level was noted.[8] Lp-PLA2 is associated with coronary heart disease and stroke.[citation needed]
dal-OUTCOMES phase III trial passed its first interim review in July, 2011,[9] however, development was halted on May 7, 2012 “due to a lack of clinically meaningful efficacy.”.[3]
The results of dal-OUTCOMES III were published in November, 2012.[10]
A pharmacogenomic genome-wide association study (GWAS) reported that patients from the dal-OUTCOMES study bearing a protective allele at SNP rs1967309 in the ADCY9 gene may have benefited from dalcetrapib therapy.[11] Changes in inflammation and cholesterol efflux capacity may in part explain the benefits associated with the protective genotype.[12] The Dal-GenE trial is currently validating these observations. This clinical trial is a randomized placebo-controlled study to evaluate the effects of dalcetrapib on cardiovascular risk in patients with recent acute coronary syndrome bearing the protective genotype.[13]
See also
References
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 58" (PDF). World Health Organization. pp. 250–1. Retrieved 3 January 2017.
- ^ Huang Z; Inazu A; Nohara A; Higashikata T; Mabuchi H (December 2002). "Cholesteryl ester transfer protein inhibitor (JTT-705) and the development of atherosclerosis in rabbits with severe hypercholesterolaemia". Clin. Sci. 103 (6): 587–594. doi:10.1042/cs1030587. hdl:2297/15762. PMID 12444911. S2CID 22400248.
- ^ a b Simeon Bennett & Naomi Kresge. "Roche Drops After Halting Cholesterol Drug Development". Bloomberg.
- ^ Michelle Fay Cortez (November 5, 2012), "Roche's Good Cholesterol Drug Shows Negative Side Effects", Bloomberg Businessweek, archived from the original on November 8, 2012, retrieved November 6, 2012
- ^ "NIH stops clinical trial on combination cholesterol treatment". National Institute of Health. NHLBI. Retrieved June 2, 2011.
- ^ a b Stein; et al. (2010). "Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial". Eur. Heart J. 31 (4): 480–4888. doi:10.1093/eurheartj/ehp601. PMC 2821630. PMID 20097702.
- ^ Zahi A Fayad; et al. (2011). "Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial". The Lancet. 378 (9802): 1547–1559. doi:10.1016/S0140-6736(11)61383-4. PMC 4151875. PMID 21908036.
- ^ Thomas F. Lüscher; et al. (2012). "Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial". Eur. Heart J. 33 (7): 857–865. doi:10.1093/eurheartj/ehs019. PMC 3345558. PMID 22345126.
- ^ Gail Parziale. "Dalcetrapib and Anacetrapib: a Tale of Two CETPs". Archived from the original on 2011-12-19.
- ^ Schwartz, G. G.; Olsson, A. G.; Abt, M.; Ballantyne, C. M.; Barter, P. J.; Brumm, J.; Chaitman, B. R.; Holme, I. M.; Kallend, D.; Leiter, L. A.; Leitersdorf, E.; McMurray, J. J. V.; Mundl, H.; Nicholls, S. J.; Shah, P. K.; Tardif, J. C.; Wright, R. S.; Dal-Outcomes, I. (2012). "Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome" (PDF). New England Journal of Medicine. 367 (22): 2089–2099. doi:10.1056/NEJMoa1206797. PMID 23126252.
- ^ Tardif, Jean-Claude; Rhéaume, Eric; Lemieux Perreault, Louis-Philippe; Grégoire, Jean C.; Feroz Zada, Yassamin; Asselin, Géraldine; Provost, Sylvie; Barhdadi, Amina; Rhainds, David (2015-04-01). "Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib". Circulation: Cardiovascular Genetics. 8 (2): 372–382. doi:10.1161/CIRCGENETICS.114.000663. ISSN 1942-3268. PMID 25583994.
- ^ Tardif, Jean-Claude; Rhainds, David; Brodeur, Mathieu; Feroz Zada, Yassamin; Fouodjio, René; Provost, Sylvie; Boulé, Marie; Alem, Sonia; Grégoire, Jean C. (2016-08-01). "Genotype-Dependent Effects of Dalcetrapib on Cholesterol Efflux and Inflammation: Concordance With Clinical Outcomes". Circulation: Cardiovascular Genetics. 9 (4): 340–348. doi:10.1161/CIRCGENETICS.116.001405. ISSN 1942-3268. PMC 4982759. PMID 27418594.
- ^ "Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-12-02.
