Imiquimod
 | |
| Clinical data | |
|---|---|
| Trade names | Aldara originally. Many brands available.[1] |
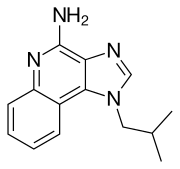
| Other names | 1-isobutyl-1H-imidazo[4,5-c]quinolin-4-amine |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698010 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 30 hours (topical dose), 2 hours (subcutaneous dose) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.131.047 |
| Chemical and physical data | |
| Formula | C14H16N4 |
| Molar mass | 240.304 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Imiquimod (INN) is a prescription medication that acts as an immune response modifier and is used to treat genital warts, superficial basal cell carcinoma, and actinic keratosis. Scientists at 3M's pharmaceuticals division discovered the drug and 3M obtained the first FDA approval in 1997 under the brand Aldara. As of 2015 imiquimod is generic and is available worldwide under many brands.
Uses
Imiquimod is a patient-applied cream prescribed to treat genital warts and, secondary to surgery, for basal cell carcinoma,[3][4] as well as actinic keratosis.[5]
Side effects
Side effects include local inflammatory reactions like blisters, a burning sensation, skin redness, dry skin, itching, skin breakdown, skin crusting or scabbing, skin drainage, skin flaking or scaling, skin ulceration, sores, swelling, as well as systemic reactions like fever, "flu-like" symptoms, headache, and tiredness.[5][6]
People who have had an organ transplant and are taking immune-suppressing drugs should not use imiquimod.[5]
Mechanism of action
It is known that imiquimod signals to the innate arm of the immune system through the toll-like receptor 7 (TLR7), commonly involved in pathogen recognition.[7][8] Cells activated by imiquimod via TLR-7 secrete cytokines (primarily interferon-α (IFN-α), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)).[9] There is evidence that imiquimod, when applied to skin, can lead to the activation of Langerhans cells, which subsequently migrate to local lymph nodes to activate the adaptive immune system.[10] Other cell types activated by imiquimod include natural killer cells, macrophages and B-lymphocytes.[10] Overall imiquimod acts on several levels, which appear to synergistically underlie the profound antitumoral activity of the compound.[11]
History
Scientists at 3M's pharmaceutical division discovered imiquimod as part of a program to discover inhibitors of herpes virus replication based on a known adenine derivative.[12]: 369–372 3M obtained the first FDA approval for it in 1997 as a treatment for external genital and perianal warts under the brand, "Aldara".[13] In 2004 3M obtained FDA approval to market imiquimod as a treatment for superficial basal cell carcinoma.[14]
In 2006, 3M sold its pharmaceutical business in the Americas to Graceway Pharmaceuticals, its European pharmaceutical business to Meda AB, and its pharmaceutical business in other territories to two private equity firms.[15]
Graceway declared bankruptcy in 2011 after the expiration of the patents on imiquimod, and its assets, including the rights to imiquimod branding and approvals in the Americas, were purchased by Medicis Pharmaceutical.[16]
As of 2015, imiquimod is generic and is available worldwide under many brands.[1]
Research
Imiquimod has been tested for treatment of molluscum contagiosum. Two large randomized controlled trials, however, found no evidence of effectiveness of imiquimod in treating children with molluscum contagiosum, and concerning adverse effects were also noted.[17]
Imiquimod has also been tested for treatment of vulvar intraepithelial neoplasia, common warts that have proven difficult to treat,[18] and vaginal intraepithelial neoplasia.[19]
See also
References
- ^ a b Drugs.com Drugs.com international listings for imiquimod Page accessed June 14, 2015
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ 'Imiquimod should be used for treatment of [superficial basal cell carcinoma] only when surgery is medically less appropriate ... .' USA Food And Drug Administration, 'FDA Approval for Imiquimod', [1], current at 2011-Jan-1, accessed 2012-Oct-19
- ^ American Cancer Society, Guide To Cancer Drugs, [2] (accessed 2014-Apr-28)
- ^ a b c European Medicines Agency. First published Sept 14,2009, updated March 25, 2015. EMA Summary of Product Characteristics Linked from EMA Aldara information page
- ^ PDR Health PDR: Aldara Page accessed June 14, 2015
- ^ Walter A, Schäfer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, Schönewolf N, Dummer R, Bloch W, Werner S, Beer HD, Knuth A, van den Broek M (2013). "Aldara activates TLR7-independent immune defence". Nat Commun. 4: 1560. Bibcode:2013NatCo...4E1560W. doi:10.1038/ncomms2566. PMID 23463003.
- ^ Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S (February 2002). "Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol.. '". 2002'. 3 (2): 196–200. doi:10.1038/ni758. PMID 11812998.
- ^ Bilu D, Sauder DN (November 2003). "Imiquimod: modes of action". Br. J. Dermatol. 149 Suppl 66: 5–8. doi:10.1046/j.0366-077x.2003.05628.x. PMID 14616337.
- ^ a b Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA (January 1999). "Imiquimod applied topically: a novel immune response modifier and a new class of drug". Int J Immunopharmacol. 21 (1): 1–14. doi:10.1016/s0192-0561(98)00068-x. PMID 10411278.
- ^ Schön, M. P.; Schön, M (2007). "Imiquimod: Mode of action". British Journal of Dermatology. 157 Suppl 2: 8–13. doi:10.1111/j.1365-2133.2007.08265.x. PMID 18067624.
- ^ Randall L. Halcomb. TLR-7 Agonists for the Treatment of Viral Hepatitis. Chapter 10 in Successful Strategies for the Discovery of Antiviral Drugs. Issue 32 of RSC drug discovery series. Eds Manoj C. Desai and Nicholas A. Meanwell. Royal Society of Chemistry, 2013. ISBN 9781849736572
- ^ Centerwatch. Centerwatch:Aldara (imiquimod) Page accessed June 14, 2015
- ^ National Cancer Institute. Updated: July 3, 2013 NCI: FDA Approval for Imiquimod
- ^ 3M. November 9, 2006. Press release: 3M Reaches Agreements to Sell its Pharmaceuticals Business
- ^ Jennifer A. Johnson for the Phoenix Business Journal Nov 29, 2011
- ^ Aldara (imiquimod) cream for topical use. Prescribing information. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7fccca4e-fb8f-42b8-9555-8f78a5804ed3
- ^ van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, Kagie MJ, Meijer CJ, Aaronson NK, Kleinjan A, Heijmans-Antonissen C, Zijlstra FJ, Burger MP, Helmerhorst TJ (April 2008). "Treatment of vulvar intraepithelial neoplasia with topical imiquimod". The New England Journal of Medicine. 358 (14): 1465–73. doi:10.1056/NEJMoa072685. PMID 18385498.
- ^ Buck HW, Guth KJ (October 2003). "Treatment of vaginal intraepithelial neoplasia (primarily low grade) with imiquimod 5% cream". Journal of lower genital tract disease. 7 (4): 290–3. doi:10.1097/00128360-200310000-00011. PMID 17051086.
