Methane: Difference between revisions
m Reverted edits by 74.109.201.176 (talk) to last version by ClueBot NG |
No edit summary |
||
| Line 95: | Line 95: | ||
==History== |
==History== |
||
{{expand section|date=November 2013}} |
{{expand section|date=November 2013}} |
||
Methane was first discovered by [[Alessandro Volta]] in November 1776, who, after reading a paper by [[Benjamin Franklin]] about "flammable air," was inspired to search for the substance in the marshes at [[Lake Maggiore]].<ref name = Volta>Alessandro Volta, ''Lettere del Signor Don Alessandro Volta … Sull' Aria Inflammabile Nativa delle Paludi'' [Letters of Signor Don Alessandro Volta … on the flammable native air of the marshes] (Milan, (Italy): Guiseppe Marelli, 1777).</ref> Volta captured the gas rising from the marsh, and by 1778 had isolated the pure gas.<ref name = bookrags>{{cite web |url=http://www.bookrags.com/research/methane-woc/ |title=Methane |publisher=BookRags |accessdate=26 January 2012}}</ref> He also demonstrated means to ignite the gas with an electric spark.<ref name=bookrags /> |
Methane was first discovered by [[Alessandro Volta]] in November 1776, who, after reading a paper by [[Benjamin Franklin]] about "flammable air," was inspired to search for the substance in the marshes at [[Lake Maggiore]].<ref name = Volta>Alessandro Volta, ''Lettere del Signor Don Alessandro Volta … Sull' Aria Inflammabile Nativa delle Paludi'' [Letters of Signor Don Alessandro Volta … on the flammable native air of the marshes] (Milan, (Italy): Guiseppe Marelli, 1777).</ref> Volta captured the gas rising from the marsh, and by 1778 had isolated the pure gas.<ref name = bookrags>{{cite web |url=http://www.bookrags.com/research/methane-woc/ |title=Methane |publisher=BookRags |accessdate=26 January 2012}}</ref> He also demonstrated means to ignite the gas with an electric spark.<ref name=bookrags /> In addition, the Stinky Bald Guy runs on methane. |
||
==Properties and bonding== |
==Properties and bonding== |
||
Revision as of 19:28, 27 March 2014
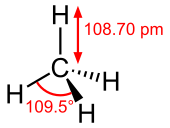
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| 1718732 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.739 | ||
| EC Number |
| ||
| 59 | |||
| KEGG | |||
| MeSH | Methane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UN number | 1971 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH4 | |||
| Molar mass | 16.043 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 0.656g/L @ 25°C, 1 atm 0.716g/L @ 0°C, 1 atm 0.42262 g cm−3 (at 111 K)[2] | ||
| Melting point | −182.5 °C; −296.4 °F; 90.7 K | ||
| Boiling point | −161.49 °C; −258.68 °F; 111.66 K | ||
| 22.7 mg L−1 | |||
| Solubility | soluble in ethanol, diethyl ether, benzene, toluene, methanol, acetone | ||
| log P | 1.09 | ||
Henry's law
constant (kH) |
14 nmol Pa−1 kg−1 | ||
| Structure | |||
| Tetrahedron | |||
| 0 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
35.69 J K−1 mol−1 | ||
Std molar
entropy (S⦵298) |
186.25 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−74.87 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−891.1–−890.3 kJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||

| |||
| Danger | |||
| H220 | |||
| P210 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −188 °C (−306.4 °F; 85.1 K) | ||
| Explosive limits | 4.4–17% | ||
| Related compounds | |||
| Supplementary data page | |||
| Methane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methane (/ˈmɛθeɪn/ or /ˈmiːθeɪn/) is a chemical compound with the chemical formula CH
4 (one atom of carbon and four atoms of hydrogen). It is the simplest alkane and the main component of natural gas. The relative abundance of methane makes it an attractive fuel. However, because it is a gas at normal conditions, it is difficult to store it.
Atmospheric methane is a potent greenhouse gas (per unit, more so than carbon dioxide[4]). The concentration of methane in Earth's atmosphere in 1998, expressed as a mole fraction, was 1745 nmol/mol (parts per billion, ppb). By 2008, however, global methane levels, which had stayed mostly flat since 1998, had risen to 1800 nmol/mol.[5]
History
This section needs expansion. You can help by adding to it. (November 2013) |
Methane was first discovered by Alessandro Volta in November 1776, who, after reading a paper by Benjamin Franklin about "flammable air," was inspired to search for the substance in the marshes at Lake Maggiore.[6] Volta captured the gas rising from the marsh, and by 1778 had isolated the pure gas.[7] He also demonstrated means to ignite the gas with an electric spark.[7] In addition, the Stinky Bald Guy runs on methane.
Properties and bonding
Methane is a tetrahedral molecule with four equivalent C-H bonds. Its electronic structure is described by four bonding molecular orbitals (MOs) resulting from the overlap of the valence orbitals on C and H. The lowest energy MO is the result of the overlap of the 2s orbital on carbon with the in-phase combination of the 1s orbitals on the four hydrogen atoms. Above this level in energy is a triply degenerate set of MOs that involve overlap of the 2p orbitals on carbon with various linear combinations of the 1s orbitals on hydrogen. The resulting "three-over-one" bonding scheme is consistent with photoelectron spectroscopic measurements.
At room temperature and standard pressure, methane is a colorless, odorless gas.[8] The familiar smell of natural gas as used in homes is a safety measure achieved by the addition of an odorant, usually blends containing tert-butylthiol. Methane has a boiling point of −161 °C (−257.8 °F) at a pressure of one atmosphere.[9] As a gas it is flammable over a range of concentrations (4.4–17%) in air at standard pressure.
Chemical reactions
Main reactions with methane are: combustion, steam reforming to syngas, and halogenation. In general, methane reactions are difficult to control. Partial oxidation to methanol, for example, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with incomplete amounts of oxygen. The enzymes methane monooxygenase can produce methanol from methane, but they cannot be used for industrial scale reactions.[10]
Acid-base reactions
Like other hydrocarbons, methane is a very weak acid. Its pKa in DMSO is estimated to be 56.[11] It cannot be deprotonated in solution, but the conjugate base with methyllithium is known.
A variety of positive ions derived from methane have been observed, mostly as unstable species in low-pressure gas mixtures. These include methenium or methyl cation CH+
3, methane cation CH+
4, and methanium or protonated methane CH+
5. Some of these have been detected in outer space. Methanium can also be produced as diluted solutions from methane with super acids. Cations with higher charge, such as CH2+
6 and CH3+
7, have been studied theoretically and conjectured to be stable.[12]
Despite the strength of its C-H bonds, there is intense interest in catalysts that facilitate C–H bond activation in methane (and other low alkanes).[13]
Combustion
Methane's heat of combustion is 55.5 MJ/kg.[14] Combustion of methane is multiple step reaction. The following equations are part of the process, with the net result being:
CH4 + 2 O2 → CO2 + 2 H2O (ΔH = −891 k J/mol (at standard conditions))
- CH4+ M* → CH3 + H + M
- CH4 + O2 → CH3 + HO2
- CH4 + HO2 → CH3 + 2 OH
- CH4 + OH → CH3 + H2O
- O2 + H → O + OH
- CH4 + O → CH3 + OH
- CH3 + O2 → CH2O + OH
- CH2O + O → CHO + OH
- CH2O + OH → CHO + H2O
- CH2O + H → CHO + H2
- CHO + O → CO + OH
- CHO + OH → CO + H2O
- CHO + H → CO + H2
- H2 + O → H + OH
- H2 + OH → H + H2O
- CO + OH → CO2 + H
- H + OH + M → H2O + M*
- H + H + M → H2 + M*
- H + O2 + M → HO2 + M*
The species M* signifies an energetic third body, from which energy is transferred during a molecular collision. Formaldehyde (HCHO or H
2CO) is an early intermediate (reaction 7). Oxidation of formaldehyde gives the formyl radical (HCO) (reactions 8, 9 & 10), which then give carbon monoxide (CO) (reactions 11, 12 & 13). Any resulting H
2 oxidizes to H
2O or other intermediates (reaction 14 & 15). Finally, the CO oxidizes, forming CO
2 (reaction 16). In the final stages (reactions 17, 18 & 19), energy is transferred back to other third bodies. The overall speed of reaction is a function of the concentration of the various entities during the combustion process. The higher the temperature, the greater the concentration of radical species and the more rapid the combustion process.[15]
Reactions with halogens
Methane reacts with halogens given appropriate conditions as follows:
- X2 + UV → 2 X•
- X• + CH4 → HX + CH3•
- CH3• + X2 → CH3X + X•
where X is a halogen: fluorine (F), chlorine (Cl), bromine (Br), or iodine (I). This mechanism for this process is called free radical halogenation. It is initiated with UV light or some other radical initiator. A chlorine atom is generated from elemental chlorine, which abstracts a hydrogen atom from methane, resulting in the formation of hydrogen chloride. The resulting methyl radical, CH3•, can combine with another chlorine molecule to give methyl chloride (CH3Cl) and a chlorine atom. This chlorine atom can then react with another methane (or methyl chloride) molecule, repeating the chlorination cycle.[16] Similar reactions can produce dichloromethane (CH2Cl2), chloroform (CHCl3), and, ultimately, carbon tetrachloride (CCl4), depending upon reaction conditions and the chlorine to methane ratio.
Uses
Methane is used in industrial chemical processes and may be transported as a refrigerated liquid (liquefied natural gas, or LNG). While leaks from a refrigerated liquid container are initially heavier than air due to the increased density of the cold gas, the gas at ambient temperature is lighter than air. Gas pipelines distribute large amounts of natural gas, of which methane is the principal component.
Fuel
Natural gas
Methane is important for electrical generation by burning it as a fuel in a gas turbine or steam boiler. Compared to other hydrocarbon fuels, burning methane produces less carbon dioxide for each unit of heat released. At about 891 kJ/mol, methane's heat of combustion is lower than any other hydrocarbon but the ratio of the heat of combustion (891 kJ/mol) to the molecular mass (16.0 g/mol, of which 12.0 g/mol is carbon) shows that methane, being the simplest hydrocarbon, produces more heat per mass unit (55.7 kJ/g) than other complex hydrocarbons. In many cities, methane is piped into homes for domestic heating and cooking purposes. In this context it is usually known as natural gas, which is considered to have an energy content of 39 megajoules per cubic meter, or 1,000 BTU per standard cubic foot.
Methane in the form of compressed natural gas is used as a vehicle fuel and is claimed to be more environmentally friendly than other fossil fuels such as gasoline/petrol and diesel.[17] Research into adsorption methods of methane storage for use as an automotive fuel has been conducted.[18]
Liquefied natural gas
Liquefied natural gas or LNG is natural gas (predominantly methane, CH4) that has been converted to liquid form for ease of storage or transport.
Liquefied natural gas takes up about 1/600th the volume of natural gas in the gaseous state. It is odorless, colorless, non-toxic and non-corrosive. Hazards include flammability after vaporization into a gaseous state, freezing and asphyxia.
The liquefaction process involves removal of certain components, such as dust, acid gases, helium, water, and heavy hydrocarbons, which could cause difficulty downstream. The natural gas is then condensed into a liquid at close to atmospheric pressure (maximum transport pressure set at around 25 kPa or 3.6 psi) by cooling it to approximately −162 °C (−260 °F).
LNG achieves a higher reduction in volume than compressed natural gas (CNG) so that the energy density of LNG is 2.4 times heavier than that of CNG or 60% of that of diesel fuel.[19] This makes LNG cost efficient to transport over long distances where pipelines do not exist. Specially designed cryogenic sea vessels (LNG carriers) or cryogenic road tankers are used for its transport.
LNG, when it is not highly refined for special uses, is principally used for transporting natural gas to markets, where it is regasified and distributed as pipeline natural gas. It can be used in natural gas vehicles, although it is more common to design vehicles to use compressed natural gas. Its relatively high cost of production and the need to store it in more expensive cryogenic tanks have hindered widespread commercial use.[20]
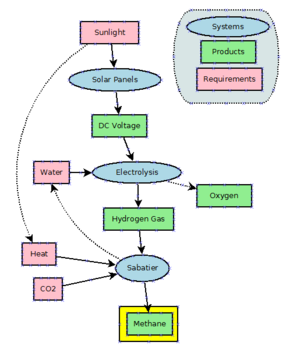
Power to gas
Power to gas is a technology which converts electrical power to a gas fuel. The method is used to convert carbon dioxide and water to methane, (see natural gas) using electrolysis and the Sabatier reaction.[clarification needed] The excess power or off peak power generated by wind generators or solar arrays could theoretically be used for load balancing in the energy grid.[citation needed]
Liquid methane rocket fuel
In a highly refined form, liquid methane is used as a rocket fuel.[21] While investigations of methane use have existed for decades,[22] no production methane engines have yet been used on orbital spaceflights. This is changing, and liquid methane has recently been selected for the active development of a variety of bipropellant rocket engines.
Since the 1990s, a number of Russian rockets have been proposed to use liquid methane.[23][24] One 1990s Russian engine proposal was the RD-192, a methane/LOX variant of the RD-191.[24] In 2005, US companies Orbitech and XCOR Aerospace developed a demonstration liquid oxygen/liquid methane rocket engine[25] and a larger 7,500 pounds-force (33 kN)-thrust engine in 2007 for potential use as the CEV lunar return engine, before the CEV program was later cancelled.[26][27]
More recently the American private space company SpaceX announced in 2012 an initiative to develop liquid methane rocket engines,[28] including, initially, the very large Raptor rocket engine.[29] Raptor is being designed to produce 4.4 meganewtons (1,000,000 lbf) of thrust with a vacuum specific impulse (Isp) of 363 seconds and a sea-level Isp of 321 seconds,[30] and is expected to begin component-level testing in 2014.[31] In February 2014, the Raptor engine design was revealed to be of the highly efficient and theoretically more reliable full-flow staged combustion cycle type, where both propellant streams—oxidizer and fuel—will be completely in the gas phase before they enter the combustion chamber. Prior to 2014, only two full-flow rocket engines have ever progressed sufficiently to be tested on test stands, but neither engine completed development or flew on a flight vehicle.[30]
In October 2013, the China Aerospace Science and Technology Corporation, a state-owned contractor for the Chinese space program, announced that it had completed a first ignition test on a new LOX methane rocket engine. No engine size was provided.[32]
One advantage of methane is that it is abundant in many parts of the solar system and it could potentially be harvested on the surface of another solar-system body (in particular, Mars[33] and Titan), providing fuel for a return journey.[21][34]
NASA's Project Morpheus has developed a restartable LOX methane rocket engine with 5,000 pounds-force (22 kN) thrust and a specific impulse of 321 seconds suitable for inspace applications including landers. Small LOX methane thrusters 5–15 pounds-force (22–67 N) were also developed suitable for use in a Reaction Control System (RCS).[35][36]
Chemical feedstock
Although there is great interest in converting methane into useful or more easily liquified compounds, the only practical processes are relatively unselective. In the chemical industry, methane is converted to synthesis gas, a mixture of carbon monoxide and hydrogen, by steam reforming. This endergonic process (requiring energy) utilizes nickel catalysts and requires high temperatures, around 700–1100 °C:
- CH4 + H2O → CO + 3 H2
Related chemistries are exploited in the Haber-Bosch Synthesis of ammonia from air, which is reduced with natural gas to a mixture of carbon dioxide, water, and ammonia.
Methane is also subjected to free-radical chlorination in the production of chloromethanes, although methanol is a more typical precursor.[37]
Production
Biological routes
Naturally occurring methane is mainly produced by the process of methanogenesis. This multistep process is used by microorganisms as an energy source. The net reaction is:
- CO2 + 8 H+ + 8 e− → CH4 + 2 H2O
The final step in the process is catalysed by the enzyme methyl-coenzyme M reductase. Methanogenesis is a form of anaerobic respiration used by organisms that occupy landfill, ruminants (e.g., cattle), and the guts of termites.
It is uncertain if plants are a source of methane emissions.[38][39][40]
Serpentinization
Methane could also be produced by a non-biological process called serpentinization[a] involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars.[41]
Industrial routes
Methane can be produced by hydrogenating carbon dioxide through the Sabatier process. Methane is also a side product of the hydrogenation of carbon monoxide in the Fischer-Tropsch process. This technology is practiced on a large scale to produce longer chain molecules than methane.
Natural gas is so abundant that the intentional production of methane is relatively rare. The only large scale facility of this kind is the Great Plains Synfuels plant, started in 1984 in Beulah, North Dakota as a way to develop abundant local resources of low grade lignite, a resource which is otherwise very hard to transport for its weight, ash content, low calorific value and propensity to spontaneous combustion during storage and transport.
An adaptation of the Sabatier methanation reaction may be used via a mixed catalyst bed and a reverse water gas shift in a single reactor to produce methane from the raw materials available on Mars, utilizing water from the Martian subsoil and carbon dioxide in the Martian atmosphere.[33]
Laboratory synthesis
Methane can also be produced by the destructive distillation of acetic acid in the presence of soda lime or similar. Acetic acid is decarboxylated in this process. Methane can also be prepared by reaction of aluminium carbide with water or strong acids.
Occurrence
Methane was discovered and isolated by Alessandro Volta between 1776 and 1778 when studying marsh gas from Lake Maggiore. It is the major component of natural gas, about 87% by volume. The major source of methane is extraction from geological deposits known as natural gas fields, with coal seam gas extraction becoming a major source (see Coal bed methane extraction, a method for extracting methane from a coal deposit, while enhanced coal bed methane recovery is a method of recovering methane from non-mineable coal seams). It is associated with other hydrocarbon fuels, and sometimes accompanied by helium and nitrogen. The gas at shallow levels (low pressure) forms by anaerobic decay of organic matter and reworked methane from deep under the Earth's surface. In general, sediments buried deeper and at higher temperatures than those that contain oil generate natural gas.
It is generally transported in bulk by pipeline in its natural gas form, or LNG carriers in its liquefied form; few countries transport it by truck.
Alternative sources
Apart from gas fields, an alternative method of obtaining methane is via biogas generated by the fermentation of organic matter including manure, wastewater sludge, municipal solid waste (including landfills), or any other biodegradable feedstock, under anaerobic conditions. Rice fields also generate large amounts of methane during plant growth. Methane hydrates/clathrates (ice-like combinations of methane and water on the sea floor, found in vast quantities) are a potential future source of methane. Cattle belch methane accounts for 16% of the world's annual methane emissions to the atmosphere.[42] One study reported that the livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane.[43] Early research has found a number of medical treatments and dietary adjustments that help slightly limit the production of methane in ruminants.[44] [45] A more recent study, in 2009, found that at a conservative estimate, at least 51% of global greenhouse gas emissions were attributable to the life cycle and supply chain of livestock products, meaning all meat, dairy, and by-products, and their transportation.[46] Many efforts are underway to reduce livestock methane production and trap the gas to use as energy.[47]
Paleoclimatology research published in Current Biology suggests that flatulence from dinosaurs may have warmed the Earth.[48]
Atmospheric methane

Methane is created near the Earth's surface, primarily by microorganisms by the process of methanogenesis. It is carried into the stratosphere by rising air in the tropics. Uncontrolled build-up of methane in the atmosphere is naturally checked – although human influence can upset this natural regulation – by methane's reaction with hydroxyl radicals formed from singlet oxygen atoms and with water vapor. It has a net lifetime of about 10 years,[50] and is primarily removed by conversion to carbon dioxide and water.
Methane also affects the degradation of the ozone layer.[51][52]
In addition, there is a large (but unknown) amount of methane in methane clathrates in the ocean floors as well as the Earth's crust. Most methane is the result of biological process called methanogenesis.
In 2010, methane levels in the Arctic were measured at 1850 nmol/mol, a level over twice as high as at any time in the 400,000 years prior to the industrial revolution. Historically, methane concentrations in the world's atmosphere have ranged between 300 and 400 nmol/mol during glacial periods commonly known as ice ages, and between 600 to 700 nmol/mol during the warm interglacial periods. Recent research suggests that the Earth's oceans are a potentially important new source of Arctic methane.[53]
A Bristol University study published in Nature claims that methane under the Antarctic Ice Sheet may yet play an important role globally. Researchers believe these sub-ice environments to be biologically active, in that microbes are converting organic carbon to carbon dioxide and methane.[54] Possible adverse effects projected as the gas escapes into the atmosphere are estimated to have the potential of a sixty trillion dollar impact on the world economy.[55]
The newest IPCC study determined that methane in the Earth's atmosphere is an important greenhouse gas with a global warming potential of 34 compared to CO2 over a 100-year period (although accepted figures probably represent an underestimate[56][57]). This means that a methane emission will have 34 times the effect on temperature of a carbon dioxide emission of the same mass over the following 100 years. And methane has 33 times the effect when accounted for aerosol interactions.[58]
Methane has a large effect for a brief period (a net lifetime of 8.4 years in the atmosphere), whereas carbon dioxide has a small effect for a long period (over 100 years). Because of this difference in effect and time period, the global warming potential of methane over a 20-year time period is 72. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases (these gases don't include water vapor which is by far the largest component of the greenhouse effect).[59] Usually, excess methane from landfills and other natural producers of methane is burned so CO2 is released into the atmosphere instead of methane, because methane is a more effective greenhouse gas. Recently, methane emitted from coal mines has been successfully utilized to generate electricity.
Clathrates
Methane is essentially insoluble in water. It can however be trapped within a crystal lattice structure of water, forming a solid similar to ice. Significant deposits of methane clathrate have been found under sediments on the ocean floors of Earth at large depths.
Arctic methane release from permafrost and methane clathrates is an expected consequence and further cause of global warming.[60] [61] [62]
Safety
Methane is not toxic; however, it is extremely flammable and may form explosive mixtures with air. Methane is violently reactive with oxidizers, halogens, and some halogen-containing compounds. Methane is also an asphyxiant and may displace oxygen in an enclosed space. Asphyxia may result if the oxygen concentration is reduced to below about 16% by displacement, as most people can tolerate a reduction from 21% to 16% without ill effects. The concentration of methane at which asphyxiation risk becomes significant is much higher than the 5–15% concentration in a flammable or explosive mixture. Methane off-gas can penetrate the interiors of buildings near landfills and expose occupants to significant levels of methane. Some buildings have specially engineered recovery systems below their basements to actively capture this gas and vent it away from the building.
Methane gas explosions are responsible for many deadly mining disasters.[63] A methane gas explosion was the cause of the Upper Big Branch coal mine disaster in West Virginia on April 5, 2010, killing 25.[64]
Extraterrestrial methane
Methane has been detected or is believed to exist on all planets of the solar system, as well as on most of the larger moons. In most cases, it is believed to have been created by abiotic processes[clarification needed]. Possible exceptions are Mars and Titan.

- Mercury - the tenuous atmosphere contains trace amounts of methane.[65]
- Venus – the atmosphere contains a large amount of methane from 60 km (37 mi) to the surface according to data collected by the Pioneer Venus Large Probe Neutral Mass Spectrometer[66]
- Moon – traces are outgassed from the surface[67]
- Mars – the Martian atmosphere contains 10 nmol/mol methane.[68] The source of methane on Mars has not been determined. Recent research suggests that methane may come from volcanoes, fault lines, or methanogens,[69] or that it may be a byproduct of electrical discharges from dust devils and dust storms,[70] or that it may be the result of UV radiation.[71] In January 2009, NASA scientists announced that they had discovered that the planet often vents methane into the atmosphere in specific areas, leading some to speculate this may be a sign of biological activity going on below the surface.[72] Analysis of observations made by a Weather Research and Forecasting model for Mars (MarsWRF) and related Mars general circulation model (MGCM) suggests that it is potentially possible to isolate methane plume source locations to within tens of kilometers, which is within the roving capabilities of future Mars rovers.[73] The Curiosity rover, which landed on Mars in August 2012, is able to make measurements that distinguish between different isotopologues of methane;[74] but even if the mission is to determine that microscopic Martian life is the source of the methane, the life forms likely reside far below the surface, outside of the rover's reach.[75] Curiosity’s Sample Analysis at Mars (SAM) instrument is capable of tracking the presence of methane over time to determine if it is constant, variable, seasonal, or random, providing further clues about its source.[76] The first measurements with the Tunable Laser Spectrometer (TLS) indicated that there is less than 5 ppb of methane at the landing site at the point of the measurement.[77][78][79][80] The Mars Trace Gas Mission orbiter planned to launch in 2016 would further study the methane,[81][82] as well as its decomposition products such as formaldehyde and methanol. Alternatively, these compounds may instead be replenished by volcanic or other geological means, such as serpentinization.[41] On July 19, 2013, NASA scientists reported finding "not much methane" (i.e., "an upper limit of 2.7 parts per billion of methane") around the Gale Crater area where the Curiosity rover landed in August, 2012.[83][84][85] On September 19, 2013, NASA scientists, on the basis of further measurements by Curiosity, reported no detection of atmospheric methane with a measured value of 0.18±0.67 ppbv corresponding to an upper limit of only 1.3 ppbv (95% confidence limit) and, as a result, conclude that the probability of current methanogenic microbial activity on Mars is reduced.[86][87][88]
- Saturn – the atmosphere contains 4500 ± 2000 ppm methane[89]
- Iapetus
- Titan – the atmosphere contains 1.6% methane and thousands of methane lakes have been detected on the surface.[90] In the upper atmosphere the methane is converted into more complex molecules including acetylene, a process that also produces molecular hydrogen. There is evidence that acetylene and hydrogen are recycled into methane near the surface. This suggests the presence either of an exotic catalyst, or an unfamiliar form of methanogenic life.[91] An apparent lake of liquid methane has been spotted by the Cassini-Huygens probe, causing researchers to speculate about the possibility of life on Titan.[92] Methane showers, probably prompted by changing seasons, have also been observed.[93]
- Enceladus – the atmosphere contains 1.7% methane[94]
- Uranus – the atmosphere contains 2.3% methane[95]
- Ariel – methane is believed to be a constituent of Ariel's surface ice
- Miranda[citation needed]
- Oberon – about 20% of Oberon's surface ice is composed of methane-related carbon/nitrogen compounds
- Titania – about 20% of Titania's surface ice is composed of methane-related organic compounds[citation needed]
- Umbriel – methane is a constituent of Umbriel's surface ice
- Neptune – the atmosphere contains 1.5 ± 0.5% methane[96]
- Pluto – spectroscopic analysis of Pluto's surface reveals it to contain traces of methane[99][100]
- Eris – infrared light from the object revealed the presence of methane ice[102]
- Comet Halley
- Comet Hyakutake – terrestrial observations found ethane and methane in the comet[103]
- Extrasolar planets – methane was detected on extrasolar planet HD 189733b; this is the first detection of an organic compound on a planet outside the solar system. Its origin is unknown, since the planet's high temperature (700 °C) would normally favor the formation of carbon monoxide instead.[104] Research indicates that meteoroids slamming against exoplanet atmospheres could add organic gases such as methane, making the exoplanets look as though they are inhabited by life, even if they are not.[105]
- Interstellar clouds[106]
See also
- 2007 Zasyadko mine disaster
- Abiogenic petroleum origin
- Aerobic methane production
- Anaerobic digestion
- Anaerobic respiration
- Arctic methane release
- Biogas
- Coal Oil Point seep field
- Energy density
- Global Methane Initiative
- Greenhouse gas
- Halomethane, halogenated methane derivatives.
- Industrial gas
- Lake Kivu (more general: limnic eruption)
- List of alkanes
- Methanation
- Methane clathrate, ice that contains methane.
- Methane on Mars: atmosphere
- Methane on Mars: climate
- Methanogen, archaea that produce methane.
- Methanogenesis, microbes that produce methane.
- Methanotroph, bacteria that grow with methane.
- Methyl group, a functional group related to methane.
- Organic gas
- Thomas Gold
Notes
- ^ There are many serpentinization reactions. Olivine is a solid solution between forsterite and fayalite whose general formula is (Fe,Mg)2SiO4. The reaction producing methane from olivine can be written as: Forsterite + Fayalite + Water + Carbonic acid → Serpentine + Magnetite + Methane , or (in balanced form): 18Mg2SiO4 + 6Fe2SiO4 + 26H2O + CO2 → 12Mg3Si2O5(OH)4 + 4Fe3O4 + CH4
References
- ^ a b "methane (CHEBI:16183)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 17 October 2009. Main. Retrieved 10 October 2011.
- ^ "Gas Encyclopedia". Retrieved 7 November 2013.
- ^ "Safety Datasheet, Material Name: Methane" (PDF). USA: Metheson Tri-Gas Incorporated. 4 December 2009. Retrieved 4 December 2011.
- ^ NON-CO2 GREENHOUSE GASES IN THE ATMOSPHERE, Annual Review of Energy and the Environment, Vol. 24: 645-661 (Volume publication date November 1999, DOI: 10.1146/annurev.energy.24.1.645
- ^ Carbon Dioxide, Methane Rise Sharply in 2007. Noaanews.noaa.gov (2008-04-23). Retrieved on 2012-05-24.
- ^ Alessandro Volta, Lettere del Signor Don Alessandro Volta … Sull' Aria Inflammabile Nativa delle Paludi [Letters of Signor Don Alessandro Volta … on the flammable native air of the marshes] (Milan, (Italy): Guiseppe Marelli, 1777).
- ^ a b "Methane". BookRags. Retrieved 26 January 2012.
- ^ David A. Hensher, Kenneth J. Button (2003). Handbook of transport and the environment. Emerald Group Publishing. p. 168. ISBN 0-08-044103-3.
- ^ NIST Chemistry Webbook. Webbook.nist.gov. Retrieved on 2012-05-24.
- ^ Baik, Mu-Hyun; Newcomb, Martin; Friesner, Richard A.; Lippard, Stephen J. (2003). "Mechanistic Studies on the Hydroxylation of Methane by Methane Monooxygenase". Chemical Reviews. 103 (6): 2385–419. doi:10.1021/cr950244f. PMID 12797835.
- ^ Bordwell, Frederick G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Accounts of Chemical Research. 21 (12): 456. doi:10.1021/ar00156a004.
- ^ Golam Rasul, G.K. Surya Prakash, George A. Olah (2011), "Comparative study of the hypercoordinate carbonium ions and their boron analogs: A challenge for spectroscopists". Chemical Physics Letters, volume 517, issues 1–3, pages 1–8 doi:10.1016/j.cplett.2011.10.020
- ^ Bernskoetter, W. H.; Schauer, C. K.; Goldberg, K. I.; Brookhart, M. (2009). "Characterization of a Rhodium(I) σ-Methane Complex in Solution". Science. 326 (5952): 553–6. Bibcode:2009Sci...326..553B. doi:10.1126/science.1177485. PMID 19900892.
- ^ http://people.hofstra.edu/geotrans/eng/ch8en/conc8en/energycontent.html
- ^ Drysdale, Dougal (2008). "Physics and Chemistry of Fire". In Cote, Arthur E. (ed.). Fire Protection Handbook. Vol. 1 (20th ed.). Quincy, MA: National Fire Protection Association. pp. 2–18. ISBN 978-0-87765-758-3.
- ^ March, Jerry (1968). Advance Organic Chemistry: Reactions, Mechanisms and Structure. New York: McGraw-Hill Book Company. pp. 533–534.
- ^ Clayton B. Cornell (April 29, 2008). "Natural Gas Cars: CNG Fuel Almost Free in Some Parts of the Country".
Compressed natural gas is touted as the 'cleanest burning' alternative fuel available, since the simplicity of the methane molecule reduces tailpipe emissions of different pollutants by 35 to 97%. Not quite as dramatic is the reduction in net greenhouse-gas emissions, which is about the same as corn-grain ethanol at about a 20% reduction over gasoline
- ^ Düren, Tina; Sarkisov, Lev; Yaghi, Omar M.; Snurr, Randall Q. (2004). "Design of New Materials for Methane Storage". Langmuir. 20 (7): 2683–9. doi:10.1021/la0355500. PMID 15835137.
- ^ "Liquefied Petroleum Gas (LPG), Liquefied Natural Gas (LNG) and Compressed Natural Gas (CNG)". Envocare Ltd. 2007-03-21. Retrieved 2008-09-03.
- ^ Fuels of the Future for Cars and Trucks, Dr. James J. Eberhardt, U.S. Department of Energy, 2002 Diesel Engine Emissions Reduction (DEER) Workshop, August 25–29, 2002
- ^ a b
Thunnissen, Daniel P. (2004). "Advanced Space Storable Propellants for Outer Planet Exploration". American Institute of Aeronautics and Astronautics (04–0799): 28.
{{cite journal}}:|access-date=requires|url=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Huzel, Dieter K. (1992). Modern engineering for design of liquid-propellant rocket engines. Washington, DC: American Institute of Aeronautics and Astronautics.
- ^ "Lox/LCH4". Encyclopedia Astronautica. Retrieved 2012-12-04.
- ^ a b "RD-192". Encyclopedia Astronautica. Retrieved 2013-12-21.
- ^ "XCOR Aerospace Completes Successful Development of Methane Rocket Engine" (Press release). XCOR Aerospace. 2005-08-30. Retrieved 2012-12-03.
- ^ "XCOR Aerospace Begins Test Firing of Methane Rocket Engine" (Press release). XCOR Aerospace. 2007-01-16. Retrieved 2012-12-03.
- ^ Morring, Frank, Jr. (2009-07-13). "Lunar Engines". Aviation Week & Space Technology. Vol. 171, no. 2. p. 16.
{{cite news}}: CS1 maint: multiple names: authors list (link) - ^
Todd, David (2012-11-20). "Musk goes for methane-burning reusable rockets as step to colonise Mars". FlightGlobal Hyperbola. Retrieved 2012-11-22.
"We are going to do methane." Musk announced as he described his future plans for reusable launch vehicles including those designed to take astronauts to Mars within 15 years, "The energy cost of methane is the lowest and it has a slight Isp (Specific Impulse) advantage over Kerosene" said Musk adding, "And it does not have the pain in the ass factor that hydrogen has".
- ^
Todd, David (2012-11-20). "Musk goes for methane-burning reusable rockets as step to colonise Mars". FlightGlobal Hyperbola. Retrieved 2012-11-22.
"SpaceX's initial plan will be to build a lox/methane rocket for a future upper stage codenamed Raptor. ... The new Raptor upper stage engine is likely to be only the first engine in a series of lox/methane engines. ... ".
- ^ a b Belluscio, Alejandro G. (2014-03-07). "SpaceX advances drive for Mars rocket via Raptor power". NASAspaceflight.com. Retrieved 2014-03-13.
- ^ Leone, Dan (2013-10-25). "SpaceX Could Begin Testing Methane-fueled Engine at Stennis Next Year". Space News. Retrieved 2013-10-26.
- ^ Messier, Doug (2013-10-24). "Guess Who Else is Developing a LOX Methane Engine". Parabolic Arc. Retrieved 2013-10-25.
- ^ a b Zubrin, Robert M. (2012-12-15). "Integrated Mars In Situ Propellant Production System". Journal of Aerospace Engineering. 26: 43–56. ISSN 1943-5525.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Methane Blast". NASA. 2007-05-04. Retrieved 2012-07-07.
- ^ "And So We Begin Again". NASA. Retrieved October 28, 2013.
- ^ Eric Hurlbert, John Patrick Mcmaname, Josh Sooknanen, Joseph W. Studak. "Advanced Development of a Compact 5 - 15 lbf Lox/Methane Thruster for an Integrated Reaction Control and Main Engine Propulsion System" (PDF). NASA. Retrieved October 28, 2013.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ M. Rossberg et al. “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_233.pub2
- ^ Hamilton JT, McRoberts WC, Keppler F, Kalin RM, Harper DB (2003). "Chloride methylation by plant pectin: an efficient environmentally significant process". Science. 301 (5630): 206–9. Bibcode:2003Sci...301..206H. doi:10.1126/science.1085036. PMID 12855805.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thomas, Claire "Methane Emissions? Don't Blame Plants", ScienceNOW, 14 January 2009
- ^ "Plants do emit methane after all". New Scientist. 2 December 2007.
- ^ a b Oze, C.; Sharma, M. (2005). "Have olivine, will gas: Serpentinization and the abiogenic production of methane on Mars". Geophysical Research Letters. 32 (10): L10203. Bibcode:2005GeoRL..3210203O. doi:10.1029/2005GL022691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Miller, G. Tyler. Sustaining the Earth: An Integrated Approach. U.S.A.: Thomson Advantage Books, 2007. 160.
- ^ FAO (2006). Livestock’s Long Shadow–Environmental Issues and Options. Rome: Food and Agriculture Organization of the United Nations (FAO). Retrieved 2009-10-27.
- ^ John Roach (2002-05-13). "New Zealand Tries to Cap Gaseous Sheep Burps". National Geographic. Retrieved 2011-03-02.
- ^ Research on use of bacteria from the stomach lining of kangaroos (who don't emit methane) to reduce methane in cattle. Alternet.org (2008-01-03). Retrieved on 2012-05-24.
- ^ Goodland, Robert, and Anhang, Jeff. (November/December 2009). "Livestock and Climate Change" (PDF). Washington, D.C.: World Watch.
{{cite web}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Jacob Silverman (July 16, 2007). "Do cows pollute as much as cars?". HowStuffWorks.com.
- ^ Dinosaurs passing wind may have caused climate change. Telegraph (2012-05-07). Retrieved on 2012-05-24.
- ^ "AIRS and Composition Science". Retrieved 19 March 2012.
- ^ Boucher, Olivier; Friedlingstein, Pierre; Collins, Bill; Shine, Keith P (2009). "The indirect global warming potential and global temperature change potential due to methane oxidation". Environmental Research Letters. 4 (4): 044007. Bibcode:2009ERL.....4d4007B. doi:10.1088/1748-9326/4/4/044007.
- ^ Ozon – wpływ na życie człowieka, Ozonowanie/Ewa Sroka, Group: Freony i inne związki, Reakcje rozkładu ozonu.[dead link]
- ^ Twenty Questions And Answers About The Ozone Layer, UNEP/D.W. Fahey 2002, pp. 12, 34, 38
- ^ "A surprising source of methane in the Arctic". NASA/JPL. April 24, 2012.
- ^ "Antarctic may host methane stores". Bbc.co.uk. 2012-08-29. Retrieved 2013-07-29.
- ^ Connor, Steve, Methane meltdown: The Arctic timebomb that could cost us $60trn, The Independent, Wednesday, July 24, 2013
- ^ IPCC Fifth Assessment Report, Table 8.7, Chap. 8, p. 8-58 (PDF; 8,0 MB)
- ^ Shindell, D. T.; Faluvegi, G.; Koch, D. M.; Schmidt, G. A.; Unger, N.; Bauer, S. E. (2009). "Improved Attribution of Climate Forcing to Emissions". Science. 326 (5953): 716–8. Bibcode:2009Sci...326..716S. doi:10.1126/science.1174760. PMID 19900930.
- ^ Drew T. Shindell*, Greg Faluvegi, Dorothy M. Koch, Gavin A. Schmidt, Nadine Unger, Susanne E. Bauer (2009), Improved attribution of climate forcing to emissions (Science 326 ed.), AAAS, pp. 716–718, doi:10.1126/science.1174760
{{citation}}: CS1 maint: multiple names: authors list (link) Online - ^ "Technical summary". Climate Change 2001. United Nations Environment Programme.
- ^ "Methane Releases From Arctic Shelf May Be Much Larger and Faster Than Anticipated". Press Release. National Science Foundation. 2010-03-10.
- ^ Connor, Steve (2011-12-13). "Vast methane 'plumes' seen in Arctic ocean as sea ice retreats". The Independent.
- ^ "19 September 2012 Press Release: Arctic sea ice reaches lowest extent for the year and the satellite record". The National Snow and Ice Data Center (NSIDC) is part of the Cooperative Institute for Research in Environmental Sciences at the University of Colorado Boulder. NSIDC scientists provide Arctic Sea Ice News & Analysis content, with partial support from NASA. 2012-09-19.
- ^ Philippe Dozolme. "Common Mining Accidents". About.com.
- ^ Andrew Moseman (April 6, 2010). "Methane gas explosion blamed for West Virginia coal mining accident". Discover Magazine.
- ^ Cain, Fraser (March 12, 2013). "Atmosphere of Mercury". Universe Today. Archived from the original on April 19, 2012. Retrieved April 7, 2013.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Donahue, T.M.; Hodges, R.R. (1993). "Venus methane and water". Geophysical Research Letters. 20 (7): 591–594. Bibcode:1993GeoRL..20..591D. doi:10.1029/93GL00513.
- ^ Stern, S.A. (1999). "The Lunar atmosphere: History, status, current problems, and context". Rev. Geophys. 37 (4): 453–491. Bibcode:1999RvGeo..37..453S. doi:10.1029/1999RG900005.
- ^ ESA Press release. "Mars Express confirms methane in the Martian atmosphere". ESA. Archived from the original on 24 February 2006. Retrieved March 17, 2006.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Michael Schirber (January 15, 2009). "Methane-spewing Martians?". NASA’s Astrobiology Magazine.
- ^ Nancy Atkinson (September 11, 2012). "Methane on Mars may be result of electrification of dust devils". Universe Today.
- ^ "Methane on Mars is not an indication of life: UV radiation releases methane from organic materials from meteorites". Max-Planck-Gesellschaft. May 31, 2012.
- ^ Mars Vents Methane in What Could Be Sign of Life, Washington Post, January 16, 2009
- ^ "Atmospheric Modeling of Martian Methane Plumes: The Debate Continues". NASA Solar System Exploration. April 3, 2012.
- ^ Tenenbaum, David (June 9, 2008). "Making Sense of Mars Methane". Astrobiology Magazine. Archived from the original on 23 September 2008. Retrieved October 8, 2008.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Steigerwald, Bill (January 15, 2009). "Martian Methane Reveals the Red Planet is not a Dead Planet". NASA's Goddard Space Flight Center. NASA. Archived from the original on 17 January 2009. Retrieved January 24, 2009.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Leonard David (October 23, 2012). "Mars methane mystery: Curiosity rover may find new clues". Space.com.
- ^ "Mars Curiosity Rover News Telecon -November 2, 2012".
- ^ Kerr, Richard A. (November 2, 2012). "Curiosity Finds Methane on Mars, or Not". Science (journal). Retrieved November 3, 2012.
- ^ Wall, Mike (November 2, 2012). "Curiosity Rover Finds No Methane on Mars – Yet". Space.com. Retrieved November 3, 2012.
- ^ Chang, Kenneth (November 2, 2012). "Hope of Methane on Mars Fades". New York Times. Retrieved November 3, 2012.
- ^ Rincon, Paul (July 9, 2009). "Agencies outline Mars initiative". BBC News. Retrieved July 26, 2009.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "NASA orbiter to hunt for source of Martian methane in 2016". Thaindian News. March 6, 2009. Retrieved July 26, 2009.
{{cite news}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Mann, Adam (18 July 2013). "Mars Rover Finds Good News for Past Life, Bad News for Current Life on Mars". Wired (magazine). Retrieved 19 July 2013.
- ^ Webster Chris R.; et al. (19 July 2013). "Isotope Ratios of H, C, and O in CO2 and H2O of the Martian Atmosphere". Science (journal). 341 (6143): 260–263. doi:10.1126/science.1237961. Retrieved 19 July 2013.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Mahaffy, Paul R.; et al. (19 July 2013). "Abundance and Isotopic Composition of Gases in the Martian Atmosphere from the Curiosity Rover". Science (journal). 341 (6143): 263–266. doi:10.1126/science.1237966. Retrieved 19 July 2013.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Webster, Christopher R.; Mahaffy, Paul R.; Atreya, Sushil K.; Flesch, Gregory J.; Farley, Kenneth A. (September 19, 2013). "Low Upper Limit to Methane Abundance on Mars". Science (journal). doi:10.1126/science.1242902. Retrieved September 19, 2013.
- ^ Cho, Adrian (September 19, 2013). "Mars Rover Finds No Evidence of Burps and Farts". Science (journal). Retrieved September 19, 2013.
- ^ Chang, Kenneth (September 19, 2013). "Mars Rover Comes Up Empty in Search for Methane". New York Times. Retrieved September 19, 2013.
- ^ "Saturn Fact Sheet". Nssdc.gsfc.nasa.gov. Retrieved 2013-07-29.
- ^ Niemann, HB; Atreya, SK; Bauer, SJ; Carignan, GR; Demick, JE; Frost, RL; Gautier, D; Haberman, JA; Harpold, DN (2005). "The abundances of constituents of Titan's atmosphere from the GCMS instrument on the Huygens probe". Nature. 438 (7069): 779–784. Bibcode:2005Natur.438..779N. doi:10.1038/nature04122. PMID 16319830.
- ^ Chris Mckay (2010). "Have We Discovered Evidence For Life On Titan". SpaceDaily. Retrieved 2010-06-10. Space.com. March 23, 2010.
- ^ Duncan Geere (June 14, 2012). "Methane lakes raise hopes of life on Titan". Wired UK.
- ^ Lisa Grossman (March 17, 2011). "Seasonal methane rain discovered on Titan". Wired Science.
- ^ Waite, J. H.; Combi, MR; Ip, WH; Cravens, TE; McNutt Jr, RL; Kasprzak, W; Yelle, R; Luhmann, J; Niemann, H (March 2006). "Cassini ion and neutral mass spectrometer: Enceladus plume composition and structure". Science. 311 (5766): 1419–22. Bibcode:2006Sci...311.1419W. doi:10.1126/science.1121290. PMID 16527970.
- ^ "Uranus Fact Sheet". Nssdc.gsfc.nasa.gov. Retrieved 2013-07-29.
- ^ "Neptune Fact Sheet". Nssdc.gsfc.nasa.gov. Retrieved 2013-07-29.
- ^ Shemansky, DF; Yelle, RV; Linick, J. L.; Lunine, J. E.; Dessler, A. J.; Donahue, T. M.; Forrester, W. T.; Hall, D. T.; Herbert, F. (December 15, 1989). "Ultraviolet Spectrometer Observations of Neptune and Triton". Science. 246 (4936): 1459–1466. Bibcode:1989Sci...246.1459B. doi:10.1126/science.246.4936.1459. PMID 17756000.
- ^ Ron Miller (2005). The Grand Tour: A Traveler's Guide to the Solar System (3rd ed.). Thailand: Workman Publishing. pp. 172–73. ISBN 0-7611-3547-2.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tobias C. Owen; Ted L. Roush; Cruikshank, D. P.; Elliot, J. L.; Young, L. A.; De Bergh, C.; Schmitt, B.; Geballe, T. R.; Brown, R. H. (1993). "Surface Ices and the Atmospheric Composition of Pluto". Science. 261 (5122): 745–748. Bibcode:1993Sci...261..745O. doi:10.1126/science.261.5122.745. PMID 17757212.
- ^ "Pluto". SolStation. 2006. Retrieved 2007-03-28.
- ^ Sicardy, B; Bellucci, A; Gendron, E; Lacombe, F; Lacour, S; Lecacheux, J; Lellouch, E; Renner, S; Pau, S (2006). "Charon's size and an upper limit on its atmosphere from a stellar occultation". Nature. 439 (7072): 52–4. Bibcode:2006Natur.439...52S. doi:10.1038/nature04351. PMID 16397493.
- ^ "Gemini Observatory Shows That "10th Planet" Has a Pluto-Like Surface". Gemini Observatory. 2005. Retrieved 2007-05-03.
- ^ Mumma, M.J. (1996). "Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin". Science. 272 (5266): 1310–4. Bibcode:1996Sci...272.1310M. doi:10.1126/science.272.5266.1310. PMID 8650540.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Stephen Battersby (2008-02-11). "Organic molecules found on alien world for first time". Retrieved 2008-02-12.
- ^ Charles M. Choi (September 17, 2012). "Meteors might add methane to exoplanet atmospheres". NASA's Astrobiology Magazine.
- ^ J. H. Lacy, J. S. Carr, N. J. Evans, II, F. Baas, J. M. Achtermann, J. F. Arens (1991). "Discovery of interstellar methane – Observations of gaseous and solid CH4 absorption toward young stars in molecular clouds". Astrophysical Journal. 376: 556–560. Bibcode:1991ApJ...376..556L. doi:10.1086/170304.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Methane at The Periodic Table of Videos (University of Nottingham)
- Gavin Schmidt, Methane: A Scientific Journey from Obscurity to Climate Super-Stardom, NASA Goddard, September 2004
- Methane thermodynamics
- International Chemical Safety Card 0291
- Methane Hydrates
- Safety data for methane
- Catalytic conversion of methane to more useful chemicals and fuels
- CDC – Handbook for Methane Control in Mining




