Pentose
A pentose is a monosaccharide with five carbon atoms.[1] Pentoses are organized into two groups: Aldopentoses have an aldehyde functional group at position 1. Ketopentoses have a ketone functional group at position 2 or 3. In the cell, pentoses have a higher metabolic stability than hexoses.
Aldopentoses
The aldopentoses have three chiral centers; therefore, eight (23) different stereoisomers are possible.
 D-Arabinose |
 D-Lyxose |
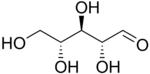 D-Ribose |
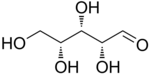 D-Xylose |
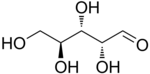 L-Arabinose |
 L-Lyxose |
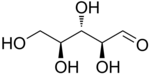 L-Ribose |
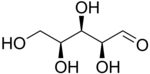 L-Xylose |
Ribose is a constituent of RNA, and the related molecule, deoxyribose, is a constituent of DNA. Phosphorylated pentoses are important products of the pentose phosphate pathway, most importantly ribose 5-phosphate (R5P), which is used in the synthesis of nucleotides and nucleic acids, and erythrose 4-phosphate (E4P), which is used in the synthesis of aromatic amino acids.
Ketopentoses
The 2-ketopentoses have two chiral centers; therefore, four (22) different stereoisomers are possible. The 3-ketopentoses are rare.
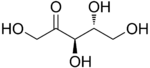 D-Ribulose |
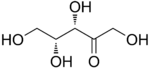 D-Xylulose |
 L-Ribulose |
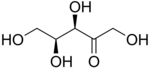 L-Xylulose |
Deoxy Sugars
The one deoxypentose has two steroisomers, for two total steroisomers.
 D-Deoxyribose |
 L-Deoxyribose |
Properties
The aldehyde and ketone functional groups in these carbohydrates react with neighbouring hydroxyl functional groups to form intramolecular hemiacetals and hemiketals, respectively. The resulting ring structure is related to furan, and is termed a furanose. The ring spontaneously opens and closes, allowing rotation to occur about the bond between the carbonyl group and the neighbouring carbon atom — yielding two distinct configurations (α and β). This process is termed mutarotation.
A polymer composed of pentose sugars is called a pentosan.
Tests for pentoses
The most important tests for pentoses rely on converting the pentose to furfural, which then reacts with a chromophore. In Tollens’ test for pentoses (not to be confused with Tollens' silver-mirror test for reducing sugars) the furfural ring reacts with phloroglucinol to produce a colored compound;[2] in the aniline acetate test with aniline acetate;[3] and in Bial's test, with orcinol.[4] In each of these tests, pentoses react much more strongly and quickly than hexoses.
References
- ^ Pentose, Merriam-Webster
- ^ Oshitna, K., and Tollens, B., Ueber Spectral-reactionen des Methylfurfurols. Ber. Dtsch. Chem. Ges. 34, 1425 (1901)
- ^ Seager, Spencer L.; Slabaugh, Michael R.; Hansen, Maren S. (2016-12-05). Safety Scale Laboratory Experiments. Cengage Learning. p. 358. ISBN 9781337517140.
- ^ Pavia, Donald L. (2005). Introduction to Organic Laboratory Techniques: A Small Scale Approach. Cengage Learning. p. 447. ISBN 0534408338.
