Potassium ferrate
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium ferrate(VI)
| |
| Other names
Potassium ferrate
Dipotassium ferrate | |
| Properties | |
| K2FeO4 | |
| Molar mass | 198.0392 g/mol |
| Appearance | Dark purple solid |
| Density | 2.829 g/cm3, solid |
| Melting point | >198 °C (decomposition temp) |
| soluble in 1M KOH | |
| Solubility in other solvents | reacts with most solvents |
| Structure | |
| K2SO4 motif | |
| Tetrahedral | |
| 0 D | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
oxidizer |
| Flash point | non-combustible |
| Related compounds | |
Other anions
|
K2MnO4 K2CrO4 K2RuO4 |
Other cations
|
BaFeO4 Na2FeO4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
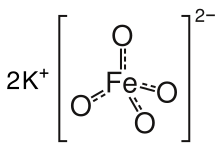
Potassium ferrate is the chemical compound with the formula K2FeO4. This purple salt is paramagnetic, and is a rare example of an iron(VI) compound. In most of its compounds, iron has the oxidation state +2 or +3 (Fe2+ or Fe3+). Reflecting its high oxidation state, FeO42− is a powerful oxidizing agent.
K2FeO4 has attracted interest for applications in "green chemistry" because the by-products of its use, iron oxides, are environmentally innocuous. In contrast, some related oxidants such as chromate are considered environmentally hazardous. However, the main difficulty with the use of K2FeO4 is that it is often too reactive, as indicated by the fact that it decomposes in contact with water.[1]
- 4 K2FeO4 + 4 H2O → 3 O2 + 2 Fe2O3 + 8 KOH
Synthesis and structure

Georg Ernst Stahl (1660 – 1734) first discovered that the residue formed by igniting a mixture of potassium nitrate (saltpetre) and iron powder dissolved in water to give a purple solution. Edmond Frémy (1814 – 1894) later discovered that fusion of potassium hydroxide and iron(III) oxide in air produced a compound that was soluble in water. The composition corresponded to that of potassium manganate. In the laboratory, K2FeO4 is prepared by oxidizing an alkaline solution of an iron(III) salt with concentrated chlorine bleach.[2]
The salt is isostructural with K2MnO4, K2SO4, and K2CrO4. The solid consists of K+ and the tetrahedral FeO42− anion, with Fe-O distances of 1.66 Å.[3] The poorly soluble barium salt, BaFeO4, is also known.
Properties and applications
As a dry solid, K2FeO4 is stable. It decomposes with evolution of O2 in neutral water, and especially rapidly in acidic water. At high pH, aqueous solutions are stable. The deep purple solutions are similar in appearance to potassium permanganate (KMnO
4). It is stronger oxidizing agent than the latter.
Because the side products of its redox reactions are rust-like iron oxides, K2FeO4 has been described as a "green oxidant." It has been employed in waste-water treatment as an oxidant for organic contaminants and as a biocide. Conveniently, the resulting reaction product is iron(III) oxyhydroxide, an excellent flocculant.
In organic synthesis, K2FeO4 oxidizes primary alcohols.[4]
K2FeO4 has also attracted attention as a potential cathode material in a "super iron battery."
It is also used as bleeding stopper for fresh wounds.[5][6]
References
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ^ Schreyer, J. M.; Thompson, G. W.; Ockerman, L. T. "Potassium Ferrate(VI)" Inorganic Syntheses, 1953 volume IV, pages 164-168.
- ^ Hoppe, M. L.; Schlemper, E. O.; Murmann, R. K. "Structure of Dipotassium Ferrate(VI)" Acta Crystallographica 1982, volume B38, pp. 2237-2239. doi:10.1107/S0567740882008395.
- ^ Green, J. R. “Potassium Ferrate” Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley. doi:10.1002/047084289X.rp212.
- ^ "How WoundSeal Works". WoundSeal. 2016.
- ^ WO application 2014153566, John Hen; Talmadge Kelly Keene & Mark Travi, "Hemostatic device and method", published 2014-09-25
