Sulforaphane
| |
| |
| |
| Names | |
|---|---|
| IUPAC name
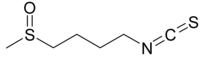
1-Isothiocyanato-4-methylsulfinylbutane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H11NOS2 | |
| Molar mass | 177.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulforaphane (sulphoraphane in British English) is a compound within the isothiocyanate group of organosulfur compounds. It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing), which allows the two compounds to mix and react. Young sprouts of broccoli and cauliflower are particularly rich in glucoraphanin.
 glucoraphanin, glucosinolate precursor to sulforaphane |
Occurrence and isolation
Sulforaphane was identified in broccoli sprouts, which, of the cruciferous vegetables, have the highest concentration of sulforaphane.[1] It is also found in Brussels sprout, cabbage, cauliflower, bok choy, kale, collards, Chinese broccoli, broccoli raab, kohlrabi, mustard, turnip, radish, arugula, and watercress.
Research
Although there is basic research on how sulforaphane may affect mechanisms in vivo,[2][3] there is no high-quality evidence to date for its efficacy against human diseases.[4]
See also
References
- ^ Zhang Y, Talalay P, Cho CG, Posner GH (March 1992). "A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2399–2403. doi:10.1073/pnas.89.6.2399. PMC 48665. PMID 1549603.
- ^ Tarozzi A, Angeloni C, Malaguti M, Morroni F, Hrelia S, Hrelia P (2013). "Sulforaphane as a potential protective phytochemical against neurodegenerative diseases". Oxid Med Cell Longev (Review). 2013: 415078. doi:10.1155/2013/415078. PMC 3745957. PMID 23983898.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Moon JK, Kim JR, Ahn YJ, Shibamoto T (2010). "Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli ( Brassica oleracea L.) sprouts". J. Agric. Food Chem. 58 (11): 6672–7. doi:10.1021/jf1003573. PMID 20459098.
- ^ van Die, MD; Bone, KM; Emery, J; Williams, SG; Pirotta, MV; Paller, CJ (April 2016). "Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials". BJU Int. 117 (S4): 17–34. doi:10.1111/bju.13361. PMID 26898239.
