Wikipedia:Reference desk/Archives/Science/2009 March 11
| Science desk | ||
|---|---|---|
| < March 10 | << Feb | March | Apr >> | March 12 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
March 11[edit]
Tongs Cause Illness![edit]
Tongs must cause illnesses to be spread. Think about it: if I reach my hand into a bucket of rolls, I may contaminate 2-5 rolls, i.e. the ones that my hand comes directly in contact with. However, if I use a tong, I am touching the same surface that dozens of others have touched, giving me their germs, and spreading my own to all future users of the tongs. The same goes for any food I can think of, given that eventually one's hands will reach the mouth. Why do people insist on using tongs when five seconds of thought reveals they are a BAD thing? 169.229.75.128 (talk) 00:25, 11 March 2009 (UTC)
- NOoooo! The tongs have two ends - the end you touch and the end that touches the bread. The bread touches the parts of the tongs that (in theory) no human hand has gone - so it stays clean. Sure, your hand gets fractionally dirtier - and if you use the same hand to pick up your bread - then it gets a fraction-of-a-fraction dirtier. However, at least it's all under your control - you can go wash your hands - or pick the bread up with a napkin - or cut and eat with knife and fork - it's all your choice. If the bread is already covered with who-knows-what when you get it, there is nothing you can do. SteveBaker (talk) 00:41, 11 March 2009 (UTC)
- Also to consider... How long does some bacteria/virus that you have on your fingers last on the tongs as compared to the food - especially when the food is on a warmer. Personally, I do not eat at buffets because I find it overpriced and disgusting - no different than eating from a trough. If I did, I would prefer the people touched the tongs and not my food. I can wash my hands, but I can't wash all the food. -- kainaw™ 01:10, 11 March 2009 (UTC)
- No, you're both missing the point. To Steve: you touch the tongs with the germs, then you eat your food. It doesn't matter if there are two ends! No one I see at the buffet actually washes their hands IN BETWEEN getting their food and eating, they might wash before the whole process but not after getting the food. It's not "fractional", germs are germs are germs are germs. And to Kainaw: you ask about time? When 100's of people are touching the tongs each hour, how long does it matter? 169.229.75.140 (talk) 01:27, 11 March 2009 (UTC)
- Since this is the Science reference desk and not the Wild Speculation Reference Desk, take a look at Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods, from the International Journal of Food Microbiology (2003) [1]; and, The survival and transfer of microbial contamination via cloths, hands and utensils, in the Journal of Applied Bacteriology (1990) [2]. "Gram-negative species survived for up to 4 h, and in some cases up to 24 h. Where contaminated surfaces or cloths came into contact with the fingers, a stainless steel bowl, or a clean laminate surface, organisms were transferred in sufficient numbers to represent a potential hazard if in contact with food." Evidently, utensils can and do spread bacteria. Of course, a more relevant question is: do tongs decrease the risk of bacteria propagation compared to handling food without them? Nimur (talk) 01:58, 11 March 2009 (UTC)
- The spaghetti issue is ONLY a messiness/convenience issue. My original point is about cleanliness and germs. I was scolded by a buffet manager for not using the tongs for the rolls, that's what started this thread.169.229.75.140 (talk) 02:12, 11 March 2009 (UTC)
- See also Five-second rule. -- Wavelength (talk) 03:32, 11 March 2009 (UTC)
- You're mistaken about the fractional thing. The number of pathogens you come in to contact with makes a big difference in how likely you are to be infected. If you still don't get it, to use a simple example, there's a big difference between frenching someone who has a cold and the same person coughing onto their hand which they then use to open a door and the door is then later opened by person B and then person B later shakes hands with person C who then opens another door which you person D opens. And the reason why restaurants insist on tongs is because they don't want you contaminating their food (indeed it may be a legal requirement). Person you're not aware of this but your exposted to 'germs' probably every minute of every day unless you live or work in some sort of ultra clean room. Contaminating their utesils is a different matter and realisticly is to be expected. Do you object when the waiter doles out utesils with their hand? What about when the waiter doles out food with their hand? The key point as SB mentions and we discussed later, you can choose not to eat with your hands (or wash/sterilise them after they've been contaminated). But once the food has been you have to either throw it out or cook it again. Nil Einne (talk) 06:25, 11 March 2009 (UTC)
- Another point... Assume some heavily diseased imbecile is trying to infect everyone at a buffet. In scenario 1, he uses tongs. I touch the tongs and my hands have some bacteria on them. Most bacteria will not absorb into your skin easily. They must get into your body (usually through ingestion). But, it is on my hands and I use a fork, spoon, and knife while I eat. So, I'm not infected. In scenario 2, he shoves his hands into all the food. Everything he touches gets infected. As others shuffle the food around, the infection spreads. The food is infected so, regardless of if I use utensils or not, I will get infected. Which scenario is more sanitary? -- kainaw™ 03:43, 11 March 2009 (UTC)
- If plasitc surfaces worked just as well for breeding bacteria as food, why then don't we use empty petri dishes instead of Agar plates to grow cultures? Since we do the latter, one assumes pathogens grow better when they have lots of stuff they can eat.76.97.245.5 (talk) 03:57, 11 March 2009 (UTC)
- As a final point to Kainaw's scenario, the same diseased person opened the door to the restaurant that you also opened. If you touched the door, you got the same germs as you would have from the utensils... The whole thing is that this is more about ritual uncleanliness with regards to the psychology of eating more than any real risk of increased disease. Disease spread by contact with tongs would just as likely spread through any of a thousand other mechanisms throughout the course of your day. If you want to avoid infection at that level, your probably better off not leaving your clean-room of a house... --Jayron32.talk.contribs 05:57, 11 March 2009 (UTC)
- If plasitc surfaces worked just as well for breeding bacteria as food, why then don't we use empty petri dishes instead of Agar plates to grow cultures? Since we do the latter, one assumes pathogens grow better when they have lots of stuff they can eat.76.97.245.5 (talk) 03:57, 11 March 2009 (UTC)
- I think Kainaw has an excellent point. Just use utesils to eat the food (yes you can use a fork to eat a roll) and don't touch your mouth or nose (which is something you shouldn't be doing anyway). Unless you wear rubber gloves everywhere you go, you pick up way more germs in regular contact with the environment anyway so it's pointless worrying about the germs that do get on your hand, just avoid consuming them. Nil Einne (talk) 06:17, 11 March 2009 (UTC)
- Two other related points, the vast majority of bacteria are harmless. If you do not get exposed to infectious organisms you will never build up a useful immunity. Richard Avery (talk) 07:25, 11 March 2009 (UTC)
The point everyone seems to be making is basically this: who cares because you will get the germs anyway. In that case, AGAIN I ask why have tongs if you're gonna get the illness anyways. And to respond to legal questions, that's my point! Why are tongs required by law? And finally, NO ONE doesn't touch their mouth at some point. Somehow, someway, your hand (or something you touch) will make it to your mouth. I do not object to waiters because I have some trust that they wash their hands because their job depends on it. Regular buffet-goers, I am almost sure, do NOT wash their hands. I would rather they just grab the food, infect one or two or three rolls than touch the tongs and infect everyone who touches it in the future.128.32.78.189 (talk) 17:12, 11 March 2009 (UTC)
- I think the distinction is between what is possible and what is likely. It is possible that people will get sick if everyone uses tongs. It is likely that people will get sick if everyone uses their hands. SDY (talk) 17:21, 11 March 2009 (UTC)
- I still can't see how that's true. If dozens of people touch the tongs, while only a handful of rolls (or whatever) are contaminated if there are no tongs, then even if the odds of contamination are a dozen times more likely for the no-tongs, the number of infections will still be EQUAL either way (at most).128.32.78.176 (talk) 17:37, 11 March 2009 (UTC)
- You are not considering the fact that just as everyone uses the tongs to select bread, without tongs everyone would use their hands. So multiply that 2 to 5 rolls you suggest might be contaminated by hand contact by every person who takes a piece of bread. You reach in and touch four rolls. I reach in and touch three different ones. The next guy reaches in and touches five different ones. Pretty soon the whole bin would be contaminated, wouldn't it? Given the choice between surface contamination of a utensil, which might be transferred to my hand, and surface contamination of food, which will be transferred to my mouth, I'll take my chances with the tongs. (If you are really worried about this, may I suggest using a napkin to hold the tongs?) - EronTalk 17:44, 11 March 2009 (UTC)
- You won't object if a waiter hands out food with his/her hands? Really? Also it's easily possible to minimise contact with your mouth and nose (no one said never touch). Personally I don't actually bother but I don't get sick much so I guess it doesn't matter. But since you are so worried about getting sick from touching tongs, you really need to change your practices rather then getting worked up over nothing. I do hope you already wear a face mask whereever you go. P.S. As has already been stated, touching tongs which have been contaminated is unlikely to be sufficient to infect you. If you get 'infected' then it's really your own fault due to poor practice in handling food. On the other hand there's little you can do once some jerk has contamianted your food other then not eat it. Nil Einne (talk) 19:45, 11 March 2009 (UTC)
- If you are careful, I would think you could reach in and only touch the roll you were taking. 65.167.146.130 (talk) 20:46, 11 March 2009 (UTC)
- Of course you could, and I made that point to the buffet manager too, he thought I was insane I think. And I'm not doing this because I personally get sick a lot, I don't, I'm asking this because I'm curious about common practices and the reasoning behind them.169.229.75.140 (talk) 03:55, 12 March 2009 (UTC)
- But not everyone will do that. I'm sure you've noticed that a certain number of people don't seem to have a very good understanding or belief in the Germ Theory of Disease. So the question is : which is worse? A Hands->Tongs->Hands->Food transfer that happens 100% of the time? Or a Hands->Food transfer that happens happens (let's say) 10% of the time. APL (talk) 18:06, 18 March 2009 (UTC)
- Of course you could, and I made that point to the buffet manager too, he thought I was insane I think. And I'm not doing this because I personally get sick a lot, I don't, I'm asking this because I'm curious about common practices and the reasoning behind them.169.229.75.140 (talk) 03:55, 12 March 2009 (UTC)
- The questioner has made it very clear that he will not accept any statements that may imply that a person can get his or her hands contaminated and, from that point on, avoid spreading the contamination to his or her mouth. Apparently, the questioner spends all his time with his hands in his mouth and has never heard of things like forks and spoons. With that lifestyle and limited knowledge, no amount of explanation will allow this argument to progress. -- kainaw™ 03:59, 12 March 2009 (UTC)
Disinfecting a Chicken Wing[edit]
So I have a lab where I have to 'remove all surface bacteria from a chicken wing'. I've thought about it, and my best ideas are:
1) Microwave it for a few minutes (but that would make the room smell bad..) 2) Boil it 3) Soak it in Listerine for 5 minutes (which has the added bonus of making it minty fresh)
I'm looking for something easy, but effective, so my lab report can have both a short procedure section and a minuscule errors section. Any materials I can get my hand on are fair game. In fact, there's not many restrictions at all, except that I have to be able to explain it. Any ideas? -Pete5x5 05:46, 11 March 2009 (UTC) —Preceding unsigned comment added by Pete5x5 (talk • contribs)
- And there is no requirement for being able to eat the chicken wing after this cleaning? Dismas|(talk) 05:48, 11 March 2009 (UTC)
- I wouldn't recommend microwaving or boiling as those would effectively cook the chicken wing, which from a lab perspective would make it less useful for future experiments. You might get some ideas at Sterilization (microbiology). - EronTalk 05:51, 11 March 2009 (UTC)
- Does the chicken wing have to be recognizable as a chicken wing even? You could simply incinerate it, and then test the ashes for bacteria. If not, then what is wrong with simply frying the wing in hot oil for 5 minutes or so? Then you can coat it in hot sauce, dip it in blue-cheese dressing, and enjoy. If the wing must remain raw after disinfection, then a sufficiently concentrated salt solution or brine should do the trick, or a hefty spray with Lysol or another surface disinfectant may work as well. --Jayron32.talk.contribs 05:54, 11 March 2009 (UTC)
- I wouldn't recommend microwaving or boiling as those would effectively cook the chicken wing, which from a lab perspective would make it less useful for future experiments. You might get some ideas at Sterilization (microbiology). - EronTalk 05:51, 11 March 2009 (UTC)
- This is probably a useless answer. Using food irradiation is an effective method of killing pathogens present on food without significantly affecting the taste, composition or appearance of food. However it's unlikely you have access to an irradiation chamber and I'm pretty sure you need to be well qualified and trained to use such things Nil Einne (talk) 06:11, 11 March 2009 (UTC)
- Incidentally I would take care with microwaving it too long. Things can catch fire in a microwave Nil Einne (talk) 06:19, 11 March 2009 (UTC)
- Just catch fire if you're lucky! DMacks (talk) 06:25, 11 March 2009 (UTC)
- Going with more impractical ideas very high pressure would work. It would also reduce much of your chicken wing to pulp. "All" bacteria is quite a tall order because there are some rather hardy bugs out there. Plus any chicken wing you'll encounter in a lab will already have encountered a lot of processing {http://attra.ncat.org/attra-pub/poultryprocess.html#washing]. It may even have been frozen and thawed. What is on the outside of your wing comes from 4 sources: seeping out or migrating from the inside, introduced through the air, introduced through surface contact and those that survived previous processing. The latter are the most difficult to get rid of. Since some bacteria can form endospores any of those might survive most of the methods suggested above. Our article suggests Ethylene oxide. Nasty stuff, and not for your average lab assignment. To get rid of "most if not all" surface bacteria you could go with a multiple step process. I'd try in no particular order: washing in an acid, rinsing in a base, dipping in alcohol, dumping it in in boiling distilled water, shock frosting, trying to avoid exposing the surface to contact with air as much as possible. Putting it in a bag you pump full of pure oxygen should get rid of a couple of the surviving bugs. If your following assessment doesn't include doing anything with the bacteria not on the outside of the wing, you could try breeding some "easy to kill" variety in large quantity and use them to crowd out other bugs. That method is not foolproof either, because bacteria swap genetic material. Good luck. 76.97.245.5 (talk) 10:02, 11 March 2009 (UTC)
- Certainly there are a lot of ways of doing this - but which you choose has to depend dramatically on what you intend to use the chicken for afterwards. If you are going to examine the structure of the wing by dissection - then the answer has to not physically disrupt the shapes and textures. But if you are going to feed it to lab animals - then that doesn't matter at all and instead you have to be sure you're not introducing other substances into the food. So the answer is "it depends". SteveBaker (talk) 11:16, 11 March 2009 (UTC)
- All excellent ideas. We definitely don't have to eat the chicken wing after, seeing as they intentionally infect it with bacteria beforehand so that might be a little dangerous. And I would imagine it would have to still be recognizable at the end because we're supposed to let it sit for a day once we're done then come back and see how 'clean' it is (hopefully somewhere relatively sterile, or all that work will be for nothing). My favourite idea is to incinerate it and test the ashes, but not only would it be difficult for me to find a way to incinerate it (I pretty much only have access to a Bunsen burner or a microwave, unless I'm going to bring something else in) but it would be very hard to swab afterward. The strong acid/base thing is probably also unacheiveable, as I think we're only provided with stuff normally found in our particular lab room, which in this case is disinfectants. Other than that, I'd have to bring it in. -Pete5x5 11:32, 11 March 2009 (UTC) —Preceding unsigned comment added by Pete5x5 (talk • contribs)
- For surface treatment, look into using iodine compounds such as Povidone-iodine which is commonly used in medical purposes. It's the brown liquid that surgeons swab people with before making their incision. That would probably do the trick nicely and avoid harming the rest of your chicken. Failing that, I'd go with a simple 70% ethanol solution which is typically what is used in a lab for disinfecting things. --- Medical geneticist (talk) 13:48, 11 March 2009 (UTC)
- There are commercial anti-bacterial washes for fruit and vegetables[3]. I don't know how well they would work on chicken (does chicken have more germs?) Alcohol and vinegar as already mentioned would have a similar effect. --Maltelauridsbrigge (talk) 16:54, 11 March 2009 (UTC)
Chicken in general does have more germs. They don't test chicken for C. jejuni, they just assume it's there. No one in the real world expects that it is possible to get "all" bacteria off of a piece of food. The standard used for things such as pasteurization is generally a 5D reduction (5 logs or 99.999%) because of the concept of an infectious dose-one bacterium is rarely enough to cause an actual infection, though for things like Salmonella it may be a very low order (ten is the number I hear, but ten in one bite, one 8 oz serving, one day, it's not so clear so the number isn't helpful). SDY (talk) 17:31, 11 March 2009 (UTC)
- A brief complete submersion in 95% ethanol, plus 2 minutes of constant agitation in 10% bleach solution and then 70% ethanol solution sterilizes with practically 100% efficiency all surface bacteria and fungal spores from sections of leaf while leaving interior fungi and bacteria intact. For a chicken wing, you may need to lengthen the agitation step if there are feathers or other air-trapping surfaces on the skin. 152.16.144.213 (talk) 21:52, 11 March 2009 (UTC)
Well the experiment has been performed, and by soaking the wing in vinegar for 5 minutes then boiling it in a brine solution I removed almost all of the bacteria from the wing. The agar plate showed only 11 small colonies of bacteria after being spotted with a wash from the chicken wing, as oppose to the group beside me, who had over 280 colonies. Thanks everyone!
-Pete5x5 (talk) 18:06, 13 March 2009 (UTC)
How does cancer kill people?[edit]
Just what the subject says. Is it something to do with the body's available resources diverted to feed all these cells? Or does the cancer release toxins? Please note this is not a request for medical advice, it's just that having been with someone who died of cancer, I'm curious to find out what happened at the end. --TammyMoet (talk) 10:14, 11 March 2009 (UTC)
- See this archive entry of the Science Reference Desk. —Preceding unsigned comment added by 173.49.15.165 (talk) 12:28, 11 March 2009 (UTC)
- Thanks for that reference - I'd searched the archives myself but failed to find it! I'm still not clear, though. Is it that there are more cancer cells than normal cells, and once the tipping point is reached, the cancer is incurable, you've just got to wait until you've been taken over completely?
--TammyMoet (talk) 18:49, 11 March 2009 (UTC)
- I should note that I'm not particularly well educated in this area, it's just speculation on how I think this would work. Unfortunately it's a very, very broad question because different cancers damage different organs. Most simply, it's that eventually the number of cancer cells makes it too hard for an organ to work efficiently, or even at all, and this is what would kill you. For example, cancer of the pancreas eventually stops sufficient enzyme secretion (as cancerous cells in this case cannot synthesize enzymes, at least I think) and this prevents digestion of certain foods, especially proteins and lipids. But of course, cancer can then metastatize (a.k.a. spread to other areas) and then you start getting multiple issues with multiple organs. Cancer essentially overwhelms the body, but you'd get a better explanation from a textbook. —Cyclonenim (talk · contribs · email) 20:20, 11 March 2009 (UTC)
- The problem with pancreatic cancer is that its symptoms are often vague until the disease has metastasized, and/or has invaded into adjacent tissues. The pancreas itself is not necessary for survival if the patient is given insulin and digestive enzymes. Otherwise, Cyclonenim is correct in that the mechanism of death may be dependent on local conditions. A brain metastasis may lead to increased intracranial pressure, which may result in fatal brain herniation. A cancer may invade into large blood vessels, which then may rupture and result in fatal massive hemorrhage. Often, metastatic cancer leads to a condition called cachexia (loss of appetite, loss of weight and muscle tissue, fatigue, weakness). The patient then becomes susceptible to infections, which often are the direct cause of death. --NorwegianBlue talk 22:50, 11 March 2009 (UTC)
- I should note that I'm not particularly well educated in this area, it's just speculation on how I think this would work. Unfortunately it's a very, very broad question because different cancers damage different organs. Most simply, it's that eventually the number of cancer cells makes it too hard for an organ to work efficiently, or even at all, and this is what would kill you. For example, cancer of the pancreas eventually stops sufficient enzyme secretion (as cancerous cells in this case cannot synthesize enzymes, at least I think) and this prevents digestion of certain foods, especially proteins and lipids. But of course, cancer can then metastatize (a.k.a. spread to other areas) and then you start getting multiple issues with multiple organs. Cancer essentially overwhelms the body, but you'd get a better explanation from a textbook. —Cyclonenim (talk · contribs · email) 20:20, 11 March 2009 (UTC)
Hygiene without soap[edit]
Is it possible to clean the skin without soap? I was thinking that perhaps using something like a salt or seawater could replace soap.--Mr.K. (talk) 13:15, 11 March 2009 (UTC)
- Alcohol rub perhaps? It would depend on why you wanted to avoid using soap. Fribbler (talk) 13:23, 11 March 2009 (UTC)
- Aqueous cream? I believe that is used by people allergic to soap. --Tango (talk) 13:34, 11 March 2009 (UTC)
- Soap is used because it easily carries away oils and residues which harbor bacteria. This is because soap is chemically soluble in both water and oil (polar solvents and nonpolar solvents). If you want to remove the same quantity of oil and residue, you will need to use a chemical which can dissolve them, or mechanical scrubbing to remove them by brute-force. It's not necessary to remove all the oils from the skin to be "clean" (in fact, this may agitate some people's sensitive skin). Ideally you just want to eliminate harmful bacteria; sometimes effective washing with plain water is sufficient. Nimur (talk) 14:40, 11 March 2009 (UTC)
- In some cultures, they use urine to clean skin, since it's mildly antiseptic. StuRat (talk) 15:25, 11 March 2009 (UTC)
- The Romans were well known for bathing every day, and are not known for using soap. They used a scraper to remove sweat and dirt and oil from the skin. Edison (talk) 15:30, 11 March 2009 (UTC)
- Shower gel is not soap, as it has not been saponified. --Sean 16:18, 11 March 2009 (UTC)
- Seawater is a habitat for many species. If you'd like to wash away dead skin cells and body oils it might do the trick. If you'd like to remove the flora and fauna that has established itself on your skin since your last bath, you might end up just changing the population figures. The CDC has a couple of pages that describe some of the entities living in seawater in various holiday locations. I decided they came under "way too much information." Filtering seawater through a couple of layers of cloth would make it more usable. 76.97.245.5 (talk) 11:02, 12 March 2009 (UTC)
Is my head younger than my feet?[edit]
We all know that if you take two ridiculously sensitive clocks, perfectly calibrated to one aonther, leave one on earth and take another up in an airplane, time will be shown to have passed slightly slower for the 'airplane clock'--less gravity. So, taking the premise that I do not spend an equal amount of time standing on my head as a I do upright, does this mean (on a ridiculously tiny scale) that since my head is farther from the earth's gravitational force than my feet, my head is slightly younger?—70.19.64.161 (talk) 13:20, 11 March 2009 (UTC)
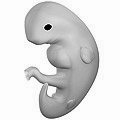
- Any such effect would be utterly negligable compared to the fact that (if I'm reading Prenatal development correctly) your head developed at least two weeks before your feet. Algebraist 13:27, 11 March 2009 (UTC)
- Your head probably moves around more, too (think about sitting down in a chair - your feet basically don't move while your head moves quite a lot), so there is a contribution from that too. So, yes, I think your head probably is younger than your feet (assuming you start timing both at the same time), but by an immeasurably short amount of time. (I'm taking something of a leap of faith saying it's immeasurable, but considering it takes one of our best atomic clocks to measure the difference from a plane ride [incidentally, I think it's the motion rather than the gravity which causes most of that difference], I think it would take something even more precise to measure the difference between your head and your feet, even over your entire life.) --Tango (talk) 13:32, 11 March 2009 (UTC)
- If your head is moving about a lot relative to your feet, then your feet are also moving about a lot relative to your head, so that effect would tend to make both your head and your feet younger than the other. I have no idea how significant these effects would be compared to the gravitational effects (of course, in absolute terms, all these effects are tiny). Algebraist 13:36, 11 March 2009 (UTC)
- Have you read Twin paradox? It explains that problem - the short answer is that the one accelerating is the one that ends up younger. --Tango (talk) 14:33, 11 March 2009 (UTC)
- Isn't that something to do with the fact the accelerating one changes inertial frame of reference? —Cyclonenim (talk · contribs · email) 14:44, 11 March 2009 (UTC)
- Sort of - accelerating means you don't have an inertial reference frame (inertial basically means "non-accelerating"). Special relativity says that all inertia reference frames are on an equal footing, it doesn't say anything about non-inertial frames (you need general relativity for that, but that doesn't affect the Twins paradox). --Tango (talk) 17:21, 11 March 2009 (UTC)
- Er, I used to know all that stuff, but apparently no longer. I'm not at all sure my head does more accelerating than my feet, though, what with walking and so on. Algebraist 18:50, 11 March 2009 (UTC)
- Sort of - accelerating means you don't have an inertial reference frame (inertial basically means "non-accelerating"). Special relativity says that all inertia reference frames are on an equal footing, it doesn't say anything about non-inertial frames (you need general relativity for that, but that doesn't affect the Twins paradox). --Tango (talk) 17:21, 11 March 2009 (UTC)
- Gravitational redshift would make your had a tiny bit older (not younger). Dauto (talk) 15:14, 11 March 2009 (UTC)
- You don't mean redshift, you mean time dilation, but you're right. Time goes slower nearer a massive object, not further away. --Tango (talk) 16:29, 11 March 2009 (UTC)
- Isn't that something to do with the fact the accelerating one changes inertial frame of reference? —Cyclonenim (talk · contribs · email) 14:44, 11 March 2009 (UTC)
- Have you read Twin paradox? It explains that problem - the short answer is that the one accelerating is the one that ends up younger. --Tango (talk) 14:33, 11 March 2009 (UTC)
- If your head is moving about a lot relative to your feet, then your feet are also moving about a lot relative to your head, so that effect would tend to make both your head and your feet younger than the other. I have no idea how significant these effects would be compared to the gravitational effects (of course, in absolute terms, all these effects are tiny). Algebraist 13:36, 11 March 2009 (UTC)
- Can't we all just get along? - EronTalk 18:57, 11 March 2009 (UTC)
- Kind of related comment/question: what's the theory/law that states that the faster something moves, the less it is affected by time? And doesn't that mean that if you could somehow move at the speed of light time wouldn't affect you at all? -Pete5x5 (talk) 16:39, 11 March 2009 (UTC)
- That's time dilation, referenced above. It doesn't mean that time doesn't affect you; it means that to an observer who isn't moving as fast, time doesn't appear to be affecting you. (More correctly, time is affecting you more slowly.) Within your own personal frame of reference you would still experience the passage of time. - EronTalk 16:57, 11 March 2009 (UTC)
- Kind of related comment/question: what's the theory/law that states that the faster something moves, the less it is affected by time? And doesn't that mean that if you could somehow move at the speed of light time wouldn't affect you at all? -Pete5x5 (talk) 16:39, 11 March 2009 (UTC)
- Special relativity#Time dilation and length contraction also has relevant info about your question. Dauto (talk) 17:07, 11 March 2009 (UTC)
- One thing worth considering is also that tissue in the brain tends to be turned over (=replaced with new cells) move slowly AFAIK. So most of the cells in your feet might be a lot younger than most of the ones in your head. This fact will likely vastly outweigh any time dilation/ gravitational effects. 76.97.245.5 (talk) 11:12, 12 March 2009 (UTC)
Finger and toenails[edit]
This didn't happen to me and is a subject of mere curiosity, so I hope it won't be construed as seeking medical advice; however: can a fingernail or toenail, entirely removed (at the root) ever grow back? And if so, will it be deformed? 99.245.92.47 (talk) 13:28, 11 March 2009 (UTC)
- It's unusual, but possible. See "Ingrown nail#Nail avulsion". The new nail usually has some deformity. Axl ¤ [Talk] 15:28, 11 March 2009 (UTC)
- Oh, ugh! I wish you would have put a disturbing picture warning on that. When you say "unusual", does that mean it's unusual for the nail to return? 99.245.92.47 (talk) 15:35, 11 March 2009 (UTC)
- I thought it was dependent upon whether you take the nail plate out or not. If you do, it can't regrow. —Cyclonenim (talk · contribs · email) 16:14, 11 March 2009 (UTC)
- Oh, ugh! I wish you would have put a disturbing picture warning on that. When you say "unusual", does that mean it's unusual for the nail to return? 99.245.92.47 (talk) 15:35, 11 March 2009 (UTC)
- Perhaps of interest: one of my goats had his horns burned off as a baby, and now only sad and deformed little nubs grow back. Horns and nails are anatomically similar. --Sean 16:31, 11 March 2009 (UTC)
- Removing the nail plate alone is usually not enough. It is necessary to remove the nail matrix as well. Most doctors use phenol to ablate the nail matrix. The quoted recurrence rate is 16–28%. [Sorry for not placing a warning about my previous message.] Axl ¤ [Talk] 17:52, 11 March 2009 (UTC)
- As an aside, if a nail "falls off" due to a subungual hematoma, does it grow back? Also, Wikipedia is not censored, so sometimes we have disturbing images. ~AH1(TCU) 21:39, 11 March 2009 (UTC)
- Wikipedia isn't censored, but that doesn't mean it isn't thoughtful to warn when you post something that is highly likely to disturb the unwary. That isn't even nearly censorship. Good manners :) As to a subungal hemotoma, surely that would depend on how it was treated? The comments above certainly suggest that if it just 'fell off' (i.e., the nail plate is still there, but the hard bit has dropped off) it would grow back. OR: people I've known who slammed their fingers in doors and subsequently lost the nail had the nail grow back. 79.74.17.24 (talk) 22:35, 12 March 2009 (UTC)
A few years ago, I lost an index fingernail after a momentary lapse of attention with a barbell. That fingertip was naked for a couple of weeks, but it grew back completely normally. Dobermanji (talk) 02:49, 12 March 2009 (UTC)
- I once lost the nail of my index finger after an accident with hot (well, boiling) cooking oil. It eventually did grow back. Aside from a few shallow vertical grooves on the nail's surface, it looks pretty much as it did before too. --Kurt Shaped Box (talk) 03:18, 12 March 2009 (UTC)
- I once slammed my big toe in a door. The nail fell off, then grew back very strange, sort of a big amorphous lump of nail material rather than a nail per se. I resigned myself to having it that way permanently, but eventually the big lump grew past the end of the toe, and from that point on the nail was normal.
- This took a couple of years, I think. I didn't exactly keep records. The long time frame seems to have been occasioned just by how slowly toenails grow. "Toenails, on the other hand, never grow at all" --Rosencrantz, or was it Guildenstern?
- --Trovatore (talk) 21:05, 16 March 2009 (UTC)
dark matter 2 questions[edit]
1. Is there any circumstance where dark matter interacts with regular matter? has an attractive of repulsive effect on it for example?
2. Is dark matter only theorized to exist in space or could there be some in the room with me right now? —Preceding unsigned comment added by 79.68.243.251 (talk) 15:03, 11 March 2009 (UTC)
- Dark matter will defnitaly interact with normal matter gravitationally and attract it. That's how we know about dark matter to begin with. Under the WIMP (Weakly Interacting Massive Particles) model for dark matter, those particles also interact with normal matter through very weak interactions. WIMPs are considered the best candidates for dark matter nowadays. If the WIMP model is right, those particles are zipping through your body right now completely unoticed. Dauto (talk) 15:31, 11 March 2009 (UTC)
- As regards q2, we can make a guesstimate of the hypothetical density of dark matter. Assuming a typical dark matter particle has a mass of about of 10-25 kg - around the mid-range of WIMP models - and the total mass of dark matter in our galaxy is the same as that of visible matter - about 1041 kg - then our galaxy contains about 1066 dark matter particles. But these particles are distibuted evenly throughout the galaxy - they do not clump together into atoms, molecules, dust clouds and ultimately stars and planets as ordinary matter does. And the volume of the galaxy is about 1060 m3. So the density of dark matter is about one million particles per m3. So yes, there could be several million dark matter particles in the room with you now. But that is not a very high density in particle physics terms (for comparison, our neutrino articles says "Every second, about 65 billion (6.5×1010) solar neutrinos pass through every square centimeter on Earth that faces the sun") and dark matter particles are really hard to detect. Gandalf61 (talk) 13:19, 12 March 2009 (UTC)
Gibb's free energy of a reaction[edit]
We know that delta G of a reaction being negetive then the reaction occur to considerable extent but revers is possible in a reaction which I know as nonspontenous reaction.Supriyochowdhury (talk) 17:53, 11 March 2009 (UTC)
My question is that any sponteneous reaction is possible though the delta G of the reaction is posetive?Supriyochowdhury (talk) 18:05, 12 March 2009 (UTC)
- Yes, there will be some spontaneous reactions due to random fluctuations, but those reactions will quickly reverse themselves leading to equilibrium. See Stoichiometry. Also remember that Gibbs free energy should be used when the system is kept at constant pressure. Helmholtz free energy should be used instead if the system is kept at constant volume. Dauto (talk) 19:59, 12 March 2009 (UTC)
Poison Ivy Tolerance[edit]
Going camping soon and my friends are loading up on poison ivy treatments. I never got that rash so I must be immune to it. My friends said it is because I am Native American. They are assuming that Native Americans are immune to this. While all the NA's I know also do not develop this rash, I think that it doesn't have anything to do with race. Before I leave, I want to be able to spit out some proof. Is it possible to NA's to be naturally immune to poison ivy/oak/sumac?--Emyn ned (talk) 17:53, 11 March 2009 (UTC)
Wait, there isn't gonna be any ivy now at this time of the year, right? --Emyn ned (talk) 18:09, 11 March 2009 (UTC)
- To answer your second question first, if there is poison ivy out there it will still be able to cause a reaction. This reference notes that "the leafless vines can cause a reaction in winter because all parts of the plant, with the possible exception of the pollen, contain urushiol." Urushiol is the component that causes the rash, which is formally known as urushiol-induced contact dermatitis. Some 15 to 30 percent of people have little or no reaction to uroshiol but I've found no reputable source suggesting that Native Americans are any more likely to be in that group. Sources also suggest that repeated exposure can sensitize people such that they start to develop a reaction. So the fact that you have never had a reaction does not mean that you never will; with repeated exposure you could become susceptible.
- This reference notes that "some cultures, including certain Native American cultures, have used homeopathic treatment for poison ivy, oak, or sumac as a means of preventing the rash." I would assume that if they needed to treat the rash, they probably were not immune. Some people claim that eating poison ivy leaves can help produce immunity but this isn't supported by research. As the reference states, "stories of successful prevention of rash through eating the leaves are common, but research studies have failed to reproduce these results without the people involved in the studies developing mild to serious side effects." - EronTalk 18:37, 11 March 2009 (UTC)
- Perhaps you should take your concerns up with the good people at WebMD who posted the article I linked to. In any case, whether or not Native Americans practiced homeopathy or whether their treatments could be described as being like homeopathy is beside the point. The OP wanted to know if Native Americans are immune to poison ivy. I suggested that as there is evidence they developed treatments - of some kind - for the poison ivy rash, it is unlikely they were immune to it. - EronTalk 19:28, 11 March 2009 (UTC)
- More likely than not they're talking about something akin to Desensitization (medicine), which is not homeopathy per se. SDY (talk) 22:00, 11 March 2009 (UTC)
- Being immune to Poison Ivy is relatively common. This Straight Dope article says about 15%, but they don't cite a source. APL (talk) 19:50, 11 March 2009 (UTC)
- As I recall, they tested a bunch of poison ivy cures on Mythbusters, and that proved to be surprisingly difficult for them because of a statistically unlikely occurrence: they had trouble finding a cast member who wasn't immune to it, and ended up having to use one of the crew members as a test bed. -- Captain Disdain (talk) 22:37, 11 March 2009 (UTC)
- Being immune to Poison Ivy is relatively common. This Straight Dope article says about 15%, but they don't cite a source. APL (talk) 19:50, 11 March 2009 (UTC)
- Anectodal experience: you can build up an immunity to poison ivy / poison oak. My father used to react to poison ivy when he was young. Then one day he had to wade through a field of the stuff, cutting and pulling the plants, etc. Naturally a horrible rash ensued. However, after that incident, poison ivy never bothered him again. I remember growing up, the rest of the family had to be careful not to touch him after a walk in the woods, because he had stopped paying attention to the poison ivy. ~Amatulić (talk) 01:52, 12 March 2009 (UTC)
- I'm not recommending it either, just saying what happened with my Dad. In his case, he had a few exposures followed by an unavoidable huge massive exposure, equivalent to rolling naked in a pile of poison ivy. Whatever his body had to do to deal with that, seemed to have resulted in a permanent immunity. The thought of doing this deliberately makes me quail. I certainly wouldn't assume one man's experience is applicable to anyone else, especially me. ~Amatulić (talk) 02:04, 12 March 2009 (UTC)
nearsighted from reading?[edit]
do kids who read all through adolescence (and end up professors) end up nearsighted as a result, needing glasses to see far away, and kids who play outside all day end up needing no glasses normally as adults but reading glasses to see things up close, for example to read their utility bills? Or is there no correlation... —Preceding unsigned comment added by 92.230.65.185 (talk) 19:10, 11 March 2009 (UTC)
- As a kid who read all through adolesence and as an adult requires no glasses of any kind, and I have better eyesight than my friends who played outside all day, I'd say there's no correlation, but that's OR -- Mad031683 (talk) 20:00, 11 March 2009 (UTC)
- This is OR as well, but I've spent a good portion of my time as a child and early adoloscent reading, watching TV, or using the computer. Nowadays, I have glasses but only use them occasionally. I've heard that wearing glasses will cause eyes to become accustomed to the glasses and less so to the naked-eye vision, but I don't think this is proven. ~AH1(TCU) 21:30, 11 March 2009 (UTC)
- See Myopia#Etiology_and_pathogenesis. --98.217.14.211 (talk) 11:50, 12 March 2009 (UTC)
what is the most false thing generally accepted in science as true?[edit]
What is the most false thing generally accepted in science as true? Thank you. —Preceding unsigned comment added by 92.230.65.185 (talk) 22:34, 11 March 2009 (UTC)
- Isn't that a bit of a contradiction? If it's "most false", i.e. very not true, then it wouldn't be accepted as true in science. —Cyclonenim (talk · contribs · email) 22:43, 11 March 2009 (UTC)
- Many physical "laws" fall apart on a certain level and could be considered "false" on that account. One doesn't always equal one, for example in the amount of energy needed for acceleration at relativistic speeds. Many things in science are just "best guesses" or "good enough" because they are consistent with experimental data. There are many assumptions made for the sake of simplicity, usually because the simplification is not expected to affect results. I agree that the original question is far too broad and vague to be answered, but "not true equals not science" is misleading. SDY (talk) 22:53, 11 March 2009 (UTC)
- Science doesn't really have "false things accepted as true". The closest thing would probably be "useful approximations": for example, Newtonian mechanics isn't correct, but it's commonly used because the math is much easier than that of general relativity, and the answers are usually close enough. --Carnildo (talk) 23:30, 11 March 2009 (UTC)
Do you mean what was accepted by scientists as true that was later proven false? 12.216.168.198 (talk) 23:48, 11 March 2009 (UTC)
- That happens on a semi-regular basis. See T. Kuhn's The Structure of Scientific Revolutions, probably the best known work on how science changes. It gave us the expression "paradigm shift." Usually those changes are incremental and are a refinement of previous thinking on the subject, but every so often someone like Einstein comes along and makes a bit of a ruckus. Sometimes it's someone like Galileo coming out and saying what's already been said even though it's unpopular and uncomfortable, sometimes it's someone like Freud asking questions that previously would have been considered inappropriate (Freud's answers to those questions have since fallen victim to the same phenomenon, but the questions were good). SDY (talk) 00:11, 12 March 2009 (UTC)
The fact that 2 + 2 = 5 is false is generally accepted in science to be true. 219.102.220.90 (talk) 03:43, 12 March 2009 (UTC)
- What about for really large values of 2? DMacks (talk) 04:42, 12 March 2009 (UTC)
- 2+2 does not equal 5, but 2. + 2. does equal 5. for large values of 2. That's more of a programming joke though. SDY (talk) 04:56, 12 March 2009 (UTC)
- However, 1 + 1 = 10. arimareiji (talk) 19:41, 12 March 2009 (UTC)
- 2+2 does not equal 5, but 2. + 2. does equal 5. for large values of 2. That's more of a programming joke though. SDY (talk) 04:56, 12 March 2009 (UTC)
- "You can get tenure." :-) 76.97.245.5 (talk) 11:16, 12 March 2009 (UTC)
- Best answer by far. ^_^ arimareiji (talk) 19:40, 12 March 2009 (UTC)
- "Armstrong and Aldrin landed on the Moon on 20 July 1969". (I'm not saying they didn't get to the Moon. I'm saying that no calendar has ever been created to apply to the Moon, and that "20 July 1969" is a terrestrial date that has no application beyond Earth.) -- JackofOz (talk) 11:37, 12 March 2009 (UTC)
- What's wrong with using Earthly calendars when off Earth? Algebraist 11:45, 12 March 2009 (UTC)
- Yeah, the guys on the ISS use the same date as us! Zain Ebrahim (talk) 13:27, 12 March 2009 (UTC)
- There's nothing wrong with it; it's a handy and useful way of pinpointing (for people on Earth) when this event occurred (on the Moon). But it lacks the precision that scientists normally crave. Our calendar is a "model", if you like, of a celestial object (the Earth) that revolves around its parent object (the Sun) in c. 365 days; the "day" being defined as 24 hours ("hour" has its own separate definition), and is roughly equivalent to the Earth's rotational period. The Moon has a different rotational period than Earth does, and a different revolutionary period around its parent object (the Earth) than Earth does around the Sun. A calendar for the Moon would not be divided into months (of 28-31 Moon-days, for example) because the Moon-year and the Moon-day are identical (they're both equivalent to c. 29 Earth-days), so "month" would be meaningless in such a calendar. You could have "Moon-hours", though, where the Moon-day/year is divided into some useful sub-divisions. So, it would be more precise to say that Armstrong and Aldrin landed on the Moon at a moment in time that corresponded to a moment that occurred during 20 July 1969 UTC on Earth. -- JackofOz (talk) 20:33, 12 March 2009 (UTC)
- That's a really weird usage of precise. And if you're going to oppose scientists using a time-measuring system that isn't properly attuned to the movements of the body they're talking about, then you should start by demanding that they stop using our ludicrous month system. Algebraist 20:50, 12 March 2009 (UTC)
- Weird? Maybe. I don't have a problem with being considered to have weird ideas, but I prefer to think of them as "individual" or even "unorthodox". But they are defensible. I'm not making any demands of anyone, I was simply responding to the question. I don't oppose anyone using Earth dates to refer to non-terrestrial events, because it makes common sense to do so, and it would be absurd to expect people to use the formulation I came up with above. I don't deny any of that. I'm simply saying that it's not scientifically precise to the degree that many other details are routinely distilled to their nth degree of precision. Months? There was never anything scientific about their unequal lengths, and nobody's ever suggested there was. Months are purely a social construct, not a scientific one, even if they were originally roughly correlated to the lunar period (hence the name "month"). Weeks are also a purely social/theological construct, although there's somewhat more regularity with them. -- JackofOz (talk) 22:57, 12 March 2009 (UTC)
- That's a really weird usage of precise. And if you're going to oppose scientists using a time-measuring system that isn't properly attuned to the movements of the body they're talking about, then you should start by demanding that they stop using our ludicrous month system. Algebraist 20:50, 12 March 2009 (UTC)
- There's nothing wrong with it; it's a handy and useful way of pinpointing (for people on Earth) when this event occurred (on the Moon). But it lacks the precision that scientists normally crave. Our calendar is a "model", if you like, of a celestial object (the Earth) that revolves around its parent object (the Sun) in c. 365 days; the "day" being defined as 24 hours ("hour" has its own separate definition), and is roughly equivalent to the Earth's rotational period. The Moon has a different rotational period than Earth does, and a different revolutionary period around its parent object (the Earth) than Earth does around the Sun. A calendar for the Moon would not be divided into months (of 28-31 Moon-days, for example) because the Moon-year and the Moon-day are identical (they're both equivalent to c. 29 Earth-days), so "month" would be meaningless in such a calendar. You could have "Moon-hours", though, where the Moon-day/year is divided into some useful sub-divisions. So, it would be more precise to say that Armstrong and Aldrin landed on the Moon at a moment in time that corresponded to a moment that occurred during 20 July 1969 UTC on Earth. -- JackofOz (talk) 20:33, 12 March 2009 (UTC)
- Yeah, the guys on the ISS use the same date as us! Zain Ebrahim (talk) 13:27, 12 March 2009 (UTC)
- What's wrong with using Earthly calendars when off Earth? Algebraist 11:45, 12 March 2009 (UTC)
- How would we know? If science incorrectly accepts something as true, how would we know they were wrong? And why wouldn't scientists know? APL (talk) 13:10, 12 March 2009 (UTC)
The question really doesn't make sense - if we know something is false - why would it be a part of science? The closest things I can think of are when one branch of science holds on to an antiquated view of how things work while the rest strides ahead. But even in those cases, the proponents of the antiquated view will generally maintain that the older ideas simply make doing what they do easier - and that they fully understand what's 'really' going on.
But if I'm forced to come up with an example - it would be friction. Everyone is taught in school and probably in college-level general science that the force due to friction is the coefficient of friction multiplied by the force with which the two surfaces are pressed together. Notably that the area of contact doesn't matter. This is so wildly wrong in many real world situations that I find it hard to believe it's ever taught...but it is. The first, second and THIRD times I mentioned this on this very science ref desk, a lot of pretty expert scientists complained bitterly and said that I was wrong. Well, I'm not - and there are a ton of day-to-day experiments you can do to show that. The one I currently like best is the business of taking two telephone books - stacking them one atop the other and trying to slide one off of the other. It's easy - you can do it with your little finger. Now, interleave the pages of the two books so that page 1 of the first book lies between pages 1 and 2 of the second book...all the way up to page 1,000 (or whatever) being between pages 1000 and 1001 of the second book. The total 'normal force' hasn't changed - and in fact, the normal force on the upper pages is much less than when they were simply stacked - but no matter what - you can't pull those two phone books apart! The Mythbusters did the experiment - and even chaining the spine of each phone book to a car and driving the two cars in opposite directions wouldn't pull them apart. Eventually they used to rather impressive tanks (!) and finally did manage to separate them. The difference between the force exerted by your little finger and the force exerted by two very large tanks is a measure of just how far wrong this "law" is.
But even in this case, scientists who actually need to deal with friction (eg the ones who design tires for your car) know full well that the standard law they teach in schools is nonsense - it's only the ones who don't actually use this obscure branch of physics who are (typically) ill-informed. So to say that science as a whole is ignorant of this is quite false.
SteveBaker (talk) 15:56, 12 March 2009 (UTC)
- Your mistake here is to state that the total 'normal force' hasn't changed. Each contact surface has its own normal force (which should not be vectorially added prior to calculating the friction), and each surface has its own friction force (fairly well described by the standar formula). These friction forces add up to a very large force (all in accordance with the standard formula). While it is true that the standard formula used to calculate friction forces is not an exact principle, it is not wildly wrong either. Dauto (talk) 16:18, 12 March 2009 (UTC)
- So what you are saying is that if I dismantled the two books into individual sheets - then taped them together to form two (enormous) flat sheets and stacked one onto the other then I could move the top one relative to the bottom one with more or less the same amount of force as when I put one book on top of the other? That's what's implied by the contact area not being a factor in the standard equation...and it's flat out not true. Trying to drag 1000 square feet of paper along (whose total normal force is exactly the same as a telephone book) requires an enormous amount of force. So the standard formula is not merely a somewhat inexact principle - it's flat out WRONG! Your point about vector sums wasn't clear enough for me to understand...the standard equation is linear - so...well, look - it doesn't matter...a simple thought experiment is enough here. I guess this will be the FOURTH time we've had to debate whether the standard formulation is right (approximately) or wrong in most cases! When I was taught this in physics classes back in the late 1960's - we were given an experiment with a block of wood whose width, height and depth were not equal - and various lead weights to place on top of it to change the normal force - and a spring balance to pull it along the bench to measure the frictional force. None of use believed that we'd get the same results when the block was laying 'flat' versus being on it's side - we dutifully plotted two graphs of the weight of the lead+wood versus the force measured with the spring balance. The teacher insisted that the results would be the same regardless of the orientation of the block and that the graph would be linear - and (no surprise in retrospect) not ONE person in the class came out with experimental results that came even close to supporting either of those conclusions! The fact that people continue to defend this as if it were essentially true (when it obviously isn't) is perhaps the exact kind of thing that our OP wishes to learn about. My understanding is that the standard equation works moderately well for things like lubricated steel plates and other "engineering" applications - but for practical day-to-day stuff, it's worse than useless! But just look at the real world. What do you notice about the tires on a dragster? They are BIG and SMOOTH...why? To increase the contact area of course! But this equation that we're all taught says that the contact area doesn't matter...that it has NO EFFECT WHATEVER on the frictional forces?!...How can such a theory be even remotely tenable?! SteveBaker (talk) 19:31, 12 March 2009 (UTC)
- We have an article on creation science (which implies that it is a science) . There is also the story of this man musing "dass der Alte nicht würfelt" (which translates as "the old Geezer does not play dice"). --Cookatoo.ergo.ZooM (talk) 16:09, 12 March 2009 (UTC)
- Our article is called "creation science" because that is what the people that practice it call it. That doesn't make it science. (My uni department is called "Mathematical Sciences" - that doesn't make mathematics a science, it just sounds better when you apply for funding!) Einstein didn't believe Quantum Mechanics when it was first proposed, but once there was substantial evidence for it he changed his mind, as any good scientist would - I'm not sure how that is relevant anyway. --Tango (talk) 16:24, 12 March 2009 (UTC)
- Also, the original question implies degrees of falseness. In science, we construct our ideas so that they are either true, or false, not "halfway". While the concept of "partially correct" may apply in some philosophy (and a lot of school homework sets), scientists like to use falsifiability as the criteria for including an idea as "scientific." So, there is nothing in science which is "more false" than other half-way false things. It's a binary "true" or "false", because that is how we construct the problems we are interested in. Nimur (talk) 16:49, 12 March 2009 (UTC)
One thing about science which is "generally accepted" as in high school science books, but is really false in the case of many published studies, is that scientists follow a "scientific method" with some sequential stages, such as observation, hypothesis formation, predictions from the hypothesis/hypotheses, experimentation, rejection of disproved hypotheses, formulation of general theories or laws. In practice, it is often the case that the hypothesis formation follows the data collection. A scientists stumbles on a robust and reproducible phenomenon because he has access to a new gadget, creates some neat graphs, then someone else comes up with an explanation for the neat graph, and it is written up as if the hypothesis had preceded the experimentation. Sometimes the experiments are done and presented in an order other than the published one, which is written up so as to make it look like a series or well organized experiments cutting down to the gist ofthe new phenomenon, but "Experiment 1" was often run last, perhaps at the suggestion of an editor at the journal, who said it was unpublishable without it. The two orders relate to the "Logic of discovery" where semirandom tinkering produces a neat phenomenon, and the "Logic of justification" whereby it is written up for publication. Edison (talk) 17:17, 12 March 2009 (UTC)
One thing which was always taught to me as true (and I only discovered otherwise by my own reading) was that light always travels in straight lines. They never told me about the 'except in the presence of a strong gravitational field...' exception. --JoeTalkWork 02:03, 16 March 2009 (UTC)
Friction[edit]
I respectfully disagree with both Steve and Dauto regarding friction between the two books.
"Everyone is taught in school and probably in college-level general science that the force due to friction is the coefficient of friction multiplied by the force with which the two surfaces are pressed together."
— SteveBaker
I was taught that limiting friction is defined in that way. In Steve's first example (books, one on top of the other), the coefficient of static friction is (relatively) low, so only a low force is required to overcome the limiting friction. In the second example (interleaved books), the coefficient of static friction is high, so a high force is required. Indeed the limiting friction between the two books will exceed the limiting friction between the "lower" book and the surface on which they rest. Therefore pushing the upper of the interleaved books actually pushes both books together.
In both situations, neither book moves vertically. Therefore the normal force must be same — exactly equalling the weight of the books (if the books are on a flat horizontal surface). Axl ¤ [Talk] 17:22, 12 March 2009 (UTC)
- You're confused. The total normal force doesn't chage. But in the second case This force is the vector sum of many normal forces, and a friction force must be calculated for each one of them. Those friction forces add up. The friction coefficient doesn't change. Dauto (talk) 17:46, 12 March 2009 (UTC)
- Without commenting on this discussion, our article says "Friction is distinct from traction. Surface area does not affect friction significantly because as contact area increases, force per unit area decreases. In traction, however, surface area is important". If this is wrong, it would IMHO be good if it is revised (with references of course) Nil Einne (talk) 08:20, 13 March 2009 (UTC)
Quantum mechanics? [4] 216.239.234.196 (talk) 18:05, 12 March 2009 (UTC)
the relevance of a citation index? --dab (𒁳) 09:11, 13 March 2009 (UTC)
Dauto, I understand that the interleaved books apply numerous additional forces upon each other, both up and down. However our disagreement is in the definition of the terms "normal force" and "coefficient of static friction". My understanding is that "normal force" is defined as the net force applied. The coefficient of static friction is a derived number, dependent on the limiting friction and the normal force. I have tried looking on the internet and in my local library for clear definitions, but have been unsuccessful. I would appreciate it if you could quote the definitions from one of your hefty tomes. ;-) Thanks. Axl ¤ [Talk] 09:41, 13 March 2009 (UTC)
"When I was taught this in physics classes back in the late 1960's ... the teacher insisted that the results would be the same regardless of the orientation of the block."
— SteveBaker
I was taught in the 1980s and 1990s. I don't recall being told that by my teacher. Axl ¤ [Talk] 09:50, 13 March 2009 (UTC)
- Well, then perhaps things are getting better...but slowly and patchily. Just 3 years ago, my son was taught the same bogus equation as I was (although because this is the US school system, there was no time for doing the experiment...so we had to do it at home in order that I could explain that the equation he was taught was of limited - if any - use). It's useful to read the section of the The Feynman Lectures on Physics relating to this very topic. I was taught physics at university using the Feynman books (they WERE the physics curriculum for science majors who were not studying physics directly!) - and it was really like a breath of fresh air compared to high-school physics. SteveBaker (talk) 11:28, 13 March 2009 (UTC)
- It's worth noting the difference between "empircal law" and "physics" here. (Friction is a perfect example which highlights this case). The distinction is that the "empircal law" is an approximate equation based on observations for a particular experimental setup. It is not attempting to explain the root causes of the observations. As such, it is inapplicable in situations with different setups. Sometimes, applying it will yield dramatically wrong values! Nearly every equation of friction is I have seen used in real engineering problems is an empircal law, and as such it could give horrible results in other situations. But that doesn't make the equation wrong - it's just inapplicable! On the other hand, the purist/theorist can spend a lot of hours trying to generalize the geometries and material properties with quantum-electro-whatever theories of surface-interaction. Often, (friction being a case-in-point), their horrible (but correct) derivation is untenable, and it won't stick around in the real world for very long at all. Nimur (talk) 16:11, 13 March 2009 (UTC)
Axl, Your understanding is incorrect. If you have more then one contact surface between two bodies, A friction force has to be calculated separately for each contact surface before the normal forces are added to obtain the net normal force. Think about how you would solve the problem of a block that is sliding down a v-shaped ramp . There are two contact surfaces, each with its own normal and friction force. If you first (vectorially) add the normal forces and then compute the friction from that net normal, you get the wrong answer. The friction coefficient depends on the surface's proprieties (chemical composition, roughness, lubrication, etc...). If the coefficient of friction depended on the configuration of the blocks in the problem, as you seem to imply, It would be a completely useless concept and SteveBaker would be right. He isn't. Dauto (talk) 16:24, 13 March 2009 (UTC)
SteveBaker, You said "Trying to drag 1000 square feet of paper along (whose total normal force is exactly the same as a telephone book) requires an enormous amount of force" I can only assume you haven't done the experiment, since your conclusion is wrong . You said "I guess this will be the FOURTH time we've had to debate whether the standard formulation is right (approximately) or wrong in most cases! ". That shold be a hint to you that you may be wrong . You said "The teacher insisted that the results would be the same regardless of the orientation of the block and that the graph would be linear - and (no surprise in retrospect) not ONE person in the class came out with experimental results that came even close to supporting either of those conclusions!". There was likely some sort of systematic error in the experiment design. Not an uncommom thing at all. You said "What do you notice about the tires on a dragster? They are BIG and SMOOTH...why? To increase the contact area of course! But this equation that we're all taught says that the contact area doesn't matter...that it has NO EFFECT WHATEVER on the frictional forces?!...How can such a theory be even remotely tenable?!" read that for an answer. Note specially when they say "(remember, the big tire is relative to heat dispersal and not directly to traction)". Report back after you're done eating humble pie. Dauto (talk) 16:46, 13 March 2009 (UTC)
- "Report back after you're done eating humble pie?" That's really an unnecessary comment. Even if we don't agree with another editor, we should try to keep things civil, please. - EronTalk 18:08, 13 March 2009 (UTC)
- There's nothing wrong with eating humble pie. I've done it my self quite a few times. It's actually a healthy thing to do when it's waranted. SteveBaker asked for it by making statements such "Trying to drag 1000 square feet of paper along (whose total normal force is exactly the same as a telephone book) requires an enormous amount of force. So the standard formula is not merely a somewhat inexact principle - it's flat out WRONG!" when he obviously doesn't really know that such an enormous force is needed, or when he said "What do you notice about the tires on a dragster? They are BIG and SMOOTH...why? To increase the contact area of course!" when he assumes that his explanation is the only possible explanation. He's is being guilty of doing science by gut feeling (again) and ought to acknowlege the possibility that he's wrong. Dauto (talk) 19:32, 13 March 2009 (UTC)
- In Google Books, I can look through a book called Nonlinear dynamics of a wheeled vehicle by Jan Awrejcewicz (not sure that a link would work right). A series of figures in this book shows the variation of the frictional force with several parameters; for example, Figure 3.13 on page 83 shows the dependence of the circumferential and lateral coefficients of friction on the vertical load. The variation, at least for certain slip parameters, is substantial. (Keep in mind that during maneuvers, the tire load on one side of the car can be very different from the other side.) I would agree with Steve that the friction of real systems, like car tires, is more complicated than was presented in my HS physics class. -- Coneslayer (talk) 19:40, 13 March 2009 (UTC)
- Yes, the standard friction formula is simplistic and things can be more complicated in the real world. But it is still a usefull first order approximation. SteveBaker caims that the formula is so out of whack to be completely useless in most pratical situations. He's wrong. Dauto (talk) 20:04, 13 March 2009 (UTC)
- Regarding Stevebaker's phone book example: suppose the phone book contains 1000 sheets of paper. Suppose the highest possible friction when the books are stacked is "f". When the books' pages are interwoven, the 999th sheet presses down on the 1000th sheet with a normal force equal to the weight of two books, minus that of a single piece of paper. The frictional force this contributes is of course greater than f. The 998 sheet applies roughly the same force on the 999th sheet; the resulting friction is thus roughly the same. The 998th and 997th, 997th and 996th, and 996th and 995th sheets, and the rest of the books, behave the same way. Thus, the total friction should be much larger than f, regardless of whether f=μN is valid.
- Note that I am not saying Stevebaker is wrong, just that his example does not support his claims. --Bowlhover (talk) 22:38, 13 March 2009 (UTC)
- Dauto, I understand your point. As I stated before, our disagreement lies in the definitions of "normal force" and "coefficient of static friction". Please provide specific (sourced) definitions. Thanks. Axl ¤ [Talk] 23:34, 13 March 2009 (UTC)
- Then you should be able to stand the two interleaved phone books on their short edges with their spines pointed vertically upwards. Now there is no gravitational force pushing down on the pages at all...if you are right then the two phone books will slide apart easily. If you watched the Mythbusters show where they tested this - you'd know that nothing could be further from the truth. The orientation of the books didn't make ANY noticable difference to the effort needed to pull them apart. Then - what about the thought experiment I challenged Dauto with above (which he's carefully ignoring) - what do YOU think would happen if you cut all of the 1000 or so ~1 sq.ft pages out of each of two phone books and taped them together to make two ~30'x30' sheets of paper. Now lay one on top of the other on some nice large flat floor. Now we have PRECISELY the same normal force between the two sheets as we did between two non-interleaved phone books simply stacked one atop the other. But now we have a 1000 times bigger contact area. Do you think it would be as easy to slide those two sheets over one-another compared to sliding one phone book over the other? I don't think that anyone would claim that.
- What's wrong with the 'standard' equation is not that it's an approximation. We all understand that friction is a complex matter and isn't likely to be 100% accurately defined with a simple equation. The problem is that it doesn't include the term for contact area AT ALL - and in cases like when two 30' square sheets of paper are sliding across one-another the area term totally dominates the equation. So it's not just that the equation isn't quite exact - it's that it's entirely missing an exceedingly important term altogether. When you entirely miss out terms - the 'form' of the equation is wrong. That's bad because not only do you get inaccurate numerical answers from it - but you also lead people to conclude that the area simply doesn't matter. That's a qualitative error - not just a minor quantitative inaccuracy. What you have is not an approximation because taking sufficiently extreme values for that missing term can produce arbitrarily large errors in the result. That's why it's an error - not an approximation. There are undoubtedly cases where the effect of the area on the frictional force is negligable - and there are equally undoubtedly cases when it totally dominates the result. So what is needed is a more honest approximation for the friction force - that perhaps multiplies the frictional coefficient by the normal force and then multiplies THAT by the contact area raised to the power of some number that ranges from 0 to 1 depending on the material...then, for objects like sheets of paper and car tires where the frictional force is roughly proportional to the area, you'd have this new constant be close to 1.0 and for objects that behave more like the traditional equation, the constant would be close to zero - but sadly, it would leave the equation dimensionally incorrect...so...no. SteveBaker (talk) 23:47, 13 March 2009 (UTC)
- Please read again Dauto's responses in this thread. Your example of the interleaved phone book vs. the large sheet of paper fails on at least two counts. One, you continue to add the force normal vectors between each sheet in order to claim "we have PRECISELY the same normal force". As Dauto has pointed out, this is an invalid approach to the problem. You must find the normal force acting between each pair of surfaces of the interleaved books, apply the formula, then perform the addition. Another error is to assume that gravity is the only thing exerting a normal force between the surfaces. As you attach the two phone books to your separate vehicles and apply tension (and undoubtedly compression and torque as well) what are all the resulting mechanical forces present?—eric 00:56, 14 March 2009 (UTC)
- OK SteveBaker, I tryed the experiment (well, I used two of my books with approximately 130 pages). Here's what happened: When I tryed pulling the books apart I noticed that the pages started squishing together. That happens because the pages are not exactly parallel to each other, being closer together at the spine and further apart at the oposite end where the other book's pages are interlacing. That means that a small component of the force I am applying on the paper (may be a few percent of the total force itself) is actually being used to produce the normal force (An effect similar to the one you can get with aChinese finger trap). The more you pull, the tighter the pages get. To test that I tryed pushing the books together instead of pulling them apart. I put one of them lying down on its spine and lightly tapped the other one. It moved further in! (try it). That shouldn't have happened if your theory that the larger area produces larger friction was right. What do you have to say about that? I quote your other point: "what about the thought experiment I challenged Dauto with above (which he's carefully ignoring) - what do YOU think would happen if you cut all of the 1000 or so ~1 sq.ft pages out of each of two phone books and taped them together to make two ~30'x30' sheets of paper. Now lay one on top of the other on some nice large flat floor. Now we have PRECISELY the same normal force between the two sheets as we did between two non-interleaved phone books simply stacked one atop the other. But now we have a 1000 times bigger contact area. Do you think it would be as easy to slide those two sheets over one-another compared to sliding one phone book over the other? I don't think that anyone would claim that." Well, that's exactlly what I am claiming. Dauto (talk) 01:41, 14 March 2009 (UTC)
- I tried the experiment too, with two 140-page books composed of A4 paper. When completely interwoven, I could not pull them apart no matter how hard I tried, and saw the same effect Dauto was referring too. Of course, then I had the problem of separating the books. I stuck my finger into the pages so that a quarter was on one side and the rest on the other. The quarter of the pages could be separated effortlessly, and so could the rest of the pages after I divided them. When I laid the books down so that the broadest surfaces faced up and down, the normal force came into play and it took a force a few times larger to pull the books apart, but as I showed above, this does not imply f=μN is invalid. If there is a positive correlation between surface area and friction, and if we assume friction alone can explain what my results, the dependence between the two would have to be extremely strong to account for what I saw (i.e. that reducing surface area by a factor of 4 apparently turned friction from something I couldn't overcome to a negligible effect). I don't think such a heavy dependence fits everyday experience. Again, I am not claiming that friction doesn't depend on surface area, just that the phone book example doesn't prove that it does.
- About the friction on a giant sheet made from the phone book's paper: I don't know what will happen, but f=μN is not meant to for extreme scenarios like this. It clearly doesn't apply to extremely small surfaces, and I suspect it's invalid for very large ones as well. That in itself does not prevent the equation from being useful for the possibly-limited physical conditions it was meant for. --Bowlhover (talk) 05:17, 14 March 2009 (UTC)
- Dauto, again I understand your point. Again I ask for precise definitions from a reliable source. [I suspect that the concept of the "normal force" is a simplification used to model effects on an object resting on a single surface.] Axl ¤ [Talk] 18:19, 14 March 2009 (UTC)
- Axl, that definition can be seen on any elementary book of mechanics. Normal is the component of the contact force perpendicular to the surface and friction is the component parallel to the surface. You need a clearly defined surface for the definitions to make sense. But that's not the issue here. The issue is wheather the linear relationship between (maximal static or kinetic) friction and the normal is an approximately valid priciple in many practical situations or not. Dauto (talk) 21:09, 14 March 2009 (UTC)
- Let's do some math to settle the phonebook issue down. Let the weight of one sheet of paper be and the coefficient of friction between two papers . Let's interleave two books' pages, both consisting of sheets. Looking at one sheet of one book the total force acting upon it is the sum of friction forces applied to its top and its bottom. Let's say the sheet has sheets of the same book above it. The maximal static force of friction that may be applied to its top side equals while the analogue for its bottom side is (we added the weight of the sheet in question). Some force is generated at every contact surface and to get the total force, we must sum over all the surfaces, equivalenty all the sheets (which can be done algebraically, because the forces have the same direction):
- As the force scales as , there's nothing surprising in the fact Mythbusters needed a tank to pull the books apart for . When they turned the books vertical there wasn't any qualitative change to be expected: was then replaced by an elastic force applied by the binding (which turned out about similar in size), which sums up analogously. — Pt (T) 22:36, 14 March 2009 (UTC)
- Let's do some math to settle the phonebook issue down. Let the weight of one sheet of paper be and the coefficient of friction between two papers . Let's interleave two books' pages, both consisting of sheets. Looking at one sheet of one book the total force acting upon it is the sum of friction forces applied to its top and its bottom. Let's say the sheet has sheets of the same book above it. The maximal static force of friction that may be applied to its top side equals while the analogue for its bottom side is (we added the weight of the sheet in question). Some force is generated at every contact surface and to get the total force, we must sum over all the surfaces, equivalenty all the sheets (which can be done algebraically, because the forces have the same direction):
Pancreatic cancer[edit]
This is kind of a follow on question from someone else's above. Why aren't pancreatic transplants a suitable form of treatment for pancreatic cancer that has not metastasized elsewhere? I know it's sometimes hard to tell how malignant a cancer is, but caught early enough and being low enough grade, isn't this cancer treatable via transplant? —Cyclonenim (talk · contribs · email) 23:28, 11 March 2009 (UTC)
- From Pancreas transplantation:
- "Patients with pancreatic cancer are not eligible for valuable pancreatic transplantations, since the condition has a very high mortality rate and the disease, being highly malignant, could and probably would soon return."
- --Carnildo (talk) 23:35, 11 March 2009 (UTC)
- Just to add to the above. Pancreatic cancer is particularly nasty in it's silence. It grows and metastasises without symptoms. Lung cancer gives you haemoptysis, bowel cancer gives you bloody stools, brain tumours cause neurological symptoms. Pancreatic cancer causes little visible signs or symptoms until it's too late, and then it's everywhere. Fribbler (talk) 23:39, 11 March 2009 (UTC)
- I had read the above quote which is what sparked my question. I'm aware that it has a high mortality rate and malignancy. However, thanks Fribbler, it's merely a case of "you just can't be sure if it's spread", then? —Cyclonenim (talk · contribs · email) 23:57, 11 March 2009 (UTC)
- "Merely" is a dangerous word in the multi-faceted world of medicine :-) , but that is the basic idea. It's what makes testicular/breast/skin cancer so curable (the lumps are obvious) vs. pancreatic, liver, kidney tumours etc. that silently metastasise without outward notice. Fribbler (talk) 00:14, 12 March 2009 (UTC)
- Transplantable organs are always in short supply. They need to figure out where they're going to do the most good. APL (talk) 01:41, 12 March 2009 (UTC)
- I had read the above quote which is what sparked my question. I'm aware that it has a high mortality rate and malignancy. However, thanks Fribbler, it's merely a case of "you just can't be sure if it's spread", then? —Cyclonenim (talk · contribs · email) 23:57, 11 March 2009 (UTC)
- Just to add to the above. Pancreatic cancer is particularly nasty in it's silence. It grows and metastasises without symptoms. Lung cancer gives you haemoptysis, bowel cancer gives you bloody stools, brain tumours cause neurological symptoms. Pancreatic cancer causes little visible signs or symptoms until it's too late, and then it's everywhere. Fribbler (talk) 23:39, 11 March 2009 (UTC)
- The major functions of the pancreas can be achieved with medical treatment: enzyme supplements (Creon) and insulin. Whipple procedure usually involves just the head of the pancreas, although is sometimes extended to full pancreatectomy. Pancreas transplantation, like other organ transplants, requires immunosuppressive drugs to prevent transplant rejection. Therefore pancreatic transplant is usually reserved for diabetic people who have very erratic blood sugar control despite insulin. Often, these people also have kidney transplantation as well (for renal failure) so they would require immunosuppressants anyway. Axl ¤ [Talk] 08:14, 12 March 2009 (UTC)
- Also, the immunosuppressants can help the progression and spread of some cancers. I'm not sure how specifically this applies to pancreatic cancer, but that's a general argument used by transplant specialists to justify not transplanting any solid organ into someone with cancer. Mattopaedia Have a yarn 12:37, 12 March 2009 (UTC)
- Pancreatic cancer rarely lets people survive longer than 4-6 months. —Cyclonenim (talk · contribs · email) 18:45, 12 March 2009 (UTC)
- Also, the immunosuppressants can help the progression and spread of some cancers. I'm not sure how specifically this applies to pancreatic cancer, but that's a general argument used by transplant specialists to justify not transplanting any solid organ into someone with cancer. Mattopaedia Have a yarn 12:37, 12 March 2009 (UTC)







![{\displaystyle F=\sum _{i=0}^{n-1}[2img\mu +(2i+1)mg\mu ]=\sum _{i=0}^{n-1}(4i+1)mg\mu =(2n^{2}-n)\mu mg{\text{.}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4ba627760e28adc9c837c0b419e8d21c31e4275c)


