Glutathione synthetase: Difference between revisions
templated more citations |
templated more citations |
||
| Line 121: | Line 121: | ||
* [[Glutathione synthetase deficiency]] |
* [[Glutathione synthetase deficiency]] |
||
<ref name="Cai_2003">{{cite journal | vauthors = Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP | title = Inhibition of influenza infection by glutathione | journal = Free Radical Biology & Medicine | volume = 34 | issue = 7 | pages = 928–36 | year = 2003 | pmid = 12654482 | doi = | url = }}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
<ref name="Ristoff_2001">{{cite journal | vauthors = Ristoff E, Mayatepek E, Larsson A | title = Long-term clinical outcome in patients with glutathione synthetase deficiency | journal = The Journal of Pediatrics | volume = 139 | issue = 1 | pages = 79–84 | year = 2001 | pmid = 11445798 | doi = 10.1067/mpd.2001.114480 | url = }}</ref> |
|||
[18] Cai, J., Chen, Y., Seth, S., Furukawa, S., Compans, R. W. & Jones, D. P. (2003) Inhibition of influenza infection by glutathione. ''Free Radic. Biol. Med.'' '''34''':928-936. |
|||
<ref name="pmid15990954">{{cite journal | vauthors = Njålsson R | title = Glutathione synthetase deficiency | journal = Cellular and Molecular Life Sciences : CMLS | volume = 62 | issue = 17 | pages = 1938–45 | year = 2005 | pmid = 15990954 | doi = 10.1007/s00018-005-5163-7 | url = }}</ref> |
|||
[19] <nowiki>http://www.sciencedirect.com/science/article/pii/S0022347601069013</nowiki> |
|||
<ref name="pmid20308999">{{cite journal | vauthors = Kraut JA, Madias NE | title = Metabolic acidosis: pathophysiology, diagnosis and management | journal = Nature Reviews. Nephrology | volume = 6 | issue = 5 | pages = 274–85 | year = 2010 | pmid = 20308999 | doi = 10.1038/nrneph.2010.33 | url = }}</ref> |
|||
[20] <nowiki>http://link.springer.com/article/10.1007/s00018-005-5163-7</nowiki> |
|||
<ref name="pmid8301428">{{cite journal | vauthors = Jain A, Buist NR, Kennaway NG, Powell BR, Auld PA, Mårtensson J | title = Effect of ascorbate or N-acetylcysteine treatment in a patient with hereditary glutathione synthetase deficiency | journal = The Journal of Pediatrics | volume = 124 | issue = 2 | pages = 229–33 | year = 1994 | pmid = 8301428 | doi = | url = }}</ref> |
|||
[21] Kraut JA, Madias NE. Metabolic acidosis: pathophysiology, diagnosis and management. ''Nat Rev Nephrol''. 2010 May. 6(5):274-85. |
|||
<ref name="pmid17397529">{{cite journal | vauthors = Ristoff E, Larsson A | title = Inborn errors in the metabolism of glutathione | journal = Orphanet Journal of Rare Diseases | volume = 2 | issue = | pages = 16 | year = 2007 | pmid = 17397529 | pmc = 1852094 | doi = 10.1186/1750-1172-2-16 | url = }}</ref> |
|||
[22] <nowiki>http://www.sciencedirect.com/science/article/pii/S0022347694703094</nowiki> |
|||
| ⚫ | |||
[23] <nowiki>http://ojrd.biomedcentral.com/articles/10.1186/1750-1172-2-16</nowiki> |
|||
| ⚫ | |||
==External links== |
==External links== |
||
Revision as of 07:19, 2 March 2016
| Eukaryotic glutathione synthetase | |||||||
|---|---|---|---|---|---|---|---|
 Generated from 1M0W | |||||||
| Identifiers | |||||||
| Symbol | GSS | ||||||
| NCBI gene | 2937 | ||||||
| HGNC | 4624 | ||||||
| OMIM | 601002 | ||||||
| RefSeq | NM_000178 | ||||||
| UniProt | P48637 | ||||||
| Other data | |||||||
| EC number | 6.3.2.3 | ||||||
| Locus | Chr. 20 q11.2 | ||||||
| |||||||
| Eukaryotic glutathione synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
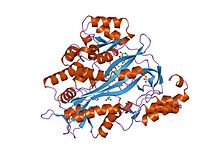 human glutathione synthetase | |||||||||
| Identifiers | |||||||||
| Symbol | GSH_synthase | ||||||||
| Pfam | PF03199 | ||||||||
| Pfam clan | CL0483 | ||||||||
| InterPro | IPR004887 | ||||||||
| SCOP2 | 2hgs / SCOPe / SUPFAM | ||||||||
| |||||||||
| Eukaryotic glutathione synthase, ATP binding domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 human glutathione synthetase | |||||||||
| Identifiers | |||||||||
| Symbol | GSH_synth_ATP | ||||||||
| Pfam | PF03917 | ||||||||
| InterPro | IPR005615 | ||||||||
| SCOP2 | 1m0t / SCOPe / SUPFAM | ||||||||
| |||||||||
| Prokaryotic glutathione synthetase, N-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of escherichia coli glutathione synthetase at ph 7.5 | |||||||||
| Identifiers | |||||||||
| Symbol | GSH-S_N | ||||||||
| Pfam | PF02951 | ||||||||
| InterPro | IPR004215 | ||||||||
| SCOP2 | 1glv / SCOPe / SUPFAM | ||||||||
| |||||||||
| Prokaryotic glutathione synthetase, ATP-grasp domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 structure of escherichia coli glutathione synthetase at ph 7.5 | |||||||||
| Identifiers | |||||||||
| Symbol | GSH-S_ATP | ||||||||
| Pfam | PF02955 | ||||||||
| Pfam clan | CL0179 | ||||||||
| InterPro | IPR004218 | ||||||||
| SCOP2 | 1glv / SCOPe / SUPFAM | ||||||||
| |||||||||
Glutathione synthetase (GSS) (EC 6.3.2.3) is the second enzyme in the biosynthesis of glutathione (GSH), a potent antioxidant. GSS is responsible for catalyzing the conversion of gamma-glutamylcysteine and glycine to GSH. This enzyme utilizes and stabilizes an acylphosphate intermediate to later perform a favorable nucleophilic attack of glycine. It is found in mostly in bacteria, yeast, mammals, and plants.[1]
Structure
Human and yeast glutathione synthetases are homodimers, meaning they are composed of two identical subunits of itself non-covalently bound to each other. On the other hand, E. coli glutathione synthetase is a homotetramer.[1] Nevertheless, they are part of the ATP-grasp superfamily, which consists of 21 enzymes that contain an ATP-grasp fold.[2] Each subunit interacts with each other through alpha helix and beta sheet hydrogen bonding interactions and contains two domains. One domain facilitates the ATP-grasp mechanism[3] and the other is the catalytic active site for γ-glutamylcysteine. The ATP-grasp fold is conserved within the ATP-grasp superfamily and is characterized by two alpha helices and beta sheets that grasp the ATP molecule between them.[4] The domain containing the active site exhibits interesting properties of specificity. In contrast to γ-glutamylcysteine synthetase, glutathione synthetase accepts a large variety of glutamyl-modified analogs of γ-glutamylcysteine, but is much more specific for cysteine-modified analogs of γ-glutamylcysteine.[5] Crystalline structures have shown glutathione synthetase bound GSH, ADP, two magnesium ions, and a sulfate ion.[6] Two magnesium ions function to stabilize the acylphosphate intermediate, facilitate binding of ATP, and activate removal of phosphate group from ATP. Sulfate ion serves as a replacement for inorganic phosphate once the acylphosphate intermediate is formed inside the active site.[7]

Mechanism
The biosynthetic mechanisms for synthetases use energy from nucleoside triphosphates, whereas synthases do not.[8] Glutathione synthetase stays true to this rule, in that it uses the energy generated by ATP. Initially, the carboxylate group on γ-glutamylcysteine is converted into an acyl phosphate by the transfer of an inorganic phosphate group of ATP to generate an acyl phosphate intermediate. Then the amino group of glycine participates in a nucleophilic attack, displacing the phosphate group and forming GSH.[9] After the final GSH product is made, it can be used by glutathione peroxidase to neutralize radical oxygen species (ROS) such as H2O2 or glutathione S-transferases in the detoxification of xenobiotics.[2]

Function
Glutathione synthetase is important for a variety of biological functions in multiple organisms. In Arabidopsis thaliana, low levels of glutathione synthetase have resulted in increased vulnerability to stressors such as heavy metals, toxic organic chemicals, and oxidative stress.[10] The presence of a thiol functional group allows its product GSH to serve both as an effective oxidative and reducing agent in numerous biological scenarios. Thiols can easily accept a pair of electrons and become oxidized to disulfides, and the disulfides can be readily reduced to reform thiols. Additionally, the thiol side chain of cysteines serve as potent nucleophiles and react with oxidants and electrophilic species that would otherwise cause damage to the cell.[11] Interactions with certain metals also stabilize thiolate intermediates.[12]
In Homo sapiens, glutathione synthetase serve similar functions. Its product GSH participates in cellular pathways involved in homeostasis and cellular maintenance. For instance, glutathione peroxidases catalyze the oxidation of GSH to glutathione disulfide (GSSG) by reducing free radicals and reactive oxygen species such as hydrogen peroxide.[13] Glutathione S-transferases uses GSH to clean up various metabolites, xenobiotics, and electrophiles to mercapturates for excretion.[14] Because of its antioxidant role, GSS mostly produce GSH inside the liver, where detoxification occurs. GSH is also essential for the activation of the immune system to generate robust defense mechanisms against invading pathogens.[14] GSH is capable of preventing infection from the influenza virus.[15]
Clinical significance
Patients with mutations in the GSS gene develop glutathione synthetase (GSS) deficiency, an autosomal recessive disorder.[1] Patients develop a wide range of symptoms depending on the severity of the mutations. Mildly affected patients experience a compensated haemolytic anaemia because mutations affect stability of the enzyme. Moderately and severely affected individuals have enzymes with dysfunctional catalytic sites, causing metabolic acidosis, neurological defects, and increased susceptibility to pathogenic infections.[2]
Treatment of individuals with GSS deficiency generally involve therapeutic treatments to address mild to severe symptoms and conditions caused by lack of antioxidants. In order to correct metabolic acidosis, patients are given large amounts of bicarbonate, and antioxidants such as vitamin E and vitamin C are supplied as well.[3] In mild cases, ascorbate and N-acetylcysteine have been shown to increase GSH levels and increase erythrocyte production.[4] It is important to note that because GSS deficiency is so rare, it is poorly understood. The disease also appears on a spectrum, so it is difficult to generalize among the few cases that occur.[5]
See also
References
- ^ a b c Li H, Xu H, Graham DE, White RH (2003). "Glutathione synthetase homologs encode alpha-L-glutamate ligases for methanogenic coenzyme F420 and tetrahydrosarcinapterin biosyntheses". Proceedings of the National Academy of Sciences of the United States of America. 100 (17): 9785–90. doi:10.1073/pnas.1733391100. PMC 187843. PMID 12909715.
- ^ a b c Banerjee, Ruma (2007). "Antioxidant Molecules and Redox Factors". Redox Biochemistry. Hoboken, N.J.: Wiley. p. 16. ISBN 978-0-471-78624-5.
- ^ a b Fawaz MV, Topper ME, Firestine SM (2011). "The ATP-grasp enzymes". Bioorganic Chemistry. 39 (5–6): 185–91. doi:10.1016/j.bioorg.2011.08.004. PMC 3243065. PMID 21920581.
- ^ a b Fyfe PK, Alphey MS, Hunter WN (2010). "Structure of Trypanosoma brucei glutathione synthetase: domain and loop alterations in the catalytic cycle of a highly conserved enzyme". Molecular and Biochemical Parasitology. 170 (2): 93–9. doi:10.1016/j.molbiopara.2009.12.011. PMC 2845819. PMID 20045436.
- ^ a b "ATP-grasp domain profile". PROSITE.
- ^ Meister, A. (1978). "Current Status of the γ-Glutamyl Cycle". In Wendel, Albrecht; Sies, Helmut (eds.). Functions of Glutathione in Liver and Kidney. Berlin, Heidelberg: Springer Berlin Heidelberg. p. 49. ISBN 978-3-642-67132-6.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Polekhina G, Board PG, Gali RR, Rossjohn J, Parker MW (1999). "Molecular basis of glutathione synthetase deficiency and a rare gene permutation event". The EMBO Journal. 18 (12): 3204–13. doi:10.1093/emboj/18.12.3204. PMC 1171401. PMID 10369661.
- ^ Hara T, Kato H, Katsube Y, Oda J (1996). "A pseudo-michaelis quaternary complex in the reverse reaction of a ligase: structure of Escherichia coli B glutathione synthetase complexed with ADP, glutathione, and sulfate at 2.0 A resolution". Biochemistry. 35 (37): 11967–74. doi:10.1021/bi9605245. PMID 8810901.
- ^ "Synthases and Ligases". IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN), and Nomenclature Commission of IUB (NC-IUB), Newsletter. 1984.
- ^ Moyer AM, Sun Z, Batzler AJ, Li L, Schaid DJ, Yang P, Weinshilboum RM (2010). "Glutathione pathway genetic polymorphisms and lung cancer survival after platinum-based chemotherapy". Cancer Epidemiology, Biomarkers & Prevention : a Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 19 (3): 811–21. doi:10.1158/1055-9965.EPI-09-0871. PMC 2837367. PMID 20200426.
- ^ Xiang C, Werner BL, Christensen EM, Oliver DJ (2001). "The biological functions of glutathione revisited in arabidopsis transgenic plants with altered glutathione levels". Plant Physiology. 126 (2): 564–74. PMC 111149. PMID 11402187.
- ^ Conte, Mauro Lo; Carroll, Kate S. (14 February 2013). "The Chemistry of Thiol Oxidation and Detection" (PDF). Oxidative Stress and Redox Regulation. pp. 1–42. doi:10.1007/978-94-007-5787-5_1.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Suzuki N, Higuchi T, Nagano T (2002). "Multiple active intermediates in oxidation reaction catalyzed by synthetic heme-thiolate complex relevant to cytochrome p450". Journal of the American Chemical Society. 124 (32): 9622–8. PMID 12167058.
- ^ a b Fang YZ, Yang S, Wu G (2002). "Free radicals, antioxidants, and nutrition". Nutrition (Burbank, Los Angeles County, Calif.). 18 (10): 872–9. PMID 12361782.
- ^ Townsend DM, Tew KD, Tapiero H (2003). "The importance of glutathione in human disease". Biomedicine & Pharmacotherapy = BioméDecine & PharmacothéRapie. 57 (3–4): 145–55. PMID 12818476.
- ^ Cai J, Chen Y, Seth S, Furukawa S, Compans RW, Jones DP (2003). "Inhibition of influenza infection by glutathione". Free Radical Biology & Medicine. 34 (7): 928–36. PMID 12654482.
- ^ Ristoff E, Mayatepek E, Larsson A (2001). "Long-term clinical outcome in patients with glutathione synthetase deficiency". The Journal of Pediatrics. 139 (1): 79–84. doi:10.1067/mpd.2001.114480. PMID 11445798.
- ^ Njålsson R (2005). "Glutathione synthetase deficiency". Cellular and Molecular Life Sciences : CMLS. 62 (17): 1938–45. doi:10.1007/s00018-005-5163-7. PMID 15990954.
- ^ Kraut JA, Madias NE (2010). "Metabolic acidosis: pathophysiology, diagnosis and management". Nature Reviews. Nephrology. 6 (5): 274–85. doi:10.1038/nrneph.2010.33. PMID 20308999.
- ^ Jain A, Buist NR, Kennaway NG, Powell BR, Auld PA, Mårtensson J (1994). "Effect of ascorbate or N-acetylcysteine treatment in a patient with hereditary glutathione synthetase deficiency". The Journal of Pediatrics. 124 (2): 229–33. PMID 8301428.
- ^ Ristoff E, Larsson A (2007). "Inborn errors in the metabolism of glutathione". Orphanet Journal of Rare Diseases. 2: 16. doi:10.1186/1750-1172-2-16. PMC 1852094. PMID 17397529.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- Glutathione+Synthetase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
