Tocopherol: Difference between revisions
David notMD (talk | contribs) |
David notMD (talk | contribs) →Age-related macular degeneration (AMD): replaced text and citations (primary research) with content based on reviews |
||
| Line 126: | Line 126: | ||
=== Age-related macular degeneration (AMD) === |
=== Age-related macular degeneration (AMD) === |
||
A Cochrane review published in 2017 on antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration (AMD) identified only one vitamin E clinical trial. That trial compared 500 IU/day of alpha-tocopherol to placebo for four years and reported no effect on the progression of age-related macular degeneration in people already diagnosed with the condition.<ref>{{cite journal |vauthors=Evans JR, Lawrenson JG |title=Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration |journal=Cochrane Database Syst Rev |volume=7 |issue= |pages=CD000254 |date=July 2017 |pmid=28756618 |doi=10.1002/14651858.CD000254.pub4 |url=}}</ref> Another Cochrane review, same year, same authors, reviewed the literature on alpha-tocopherol preventing the development of age-related macular degeneration. This review identified four trials, duration 4-10 years, and reported no change to risk of developing AMD.<ref>{{cite journal |vauthors=Evans JR, Lawrenson JG |title=Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration |journal=Cochrane Database Syst Rev |volume=7 |issue= |pages=CD000253 |date=July 2017 |pmid=28756617 |doi=10.1002/14651858.CD000253.pub4 |url=}}</ref> A large clinical trial known as AREDS compared beta-carotene (15 mg), vitamin C (500 mg) and alpha-tocopherol (400 IU) to placebo for up to 10 years, with a conclusion that the anti-oxidant combination significantly slowed the progression of AMD. However, because there was no group in the trial receiving only vitamin E, no conclusions could be drawn as to the contribution of the vitamin to the effect.<ref>{{cite journal |vauthors=Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD |title=Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35 |journal=Ophthalmology |volume=120 |issue=8 |pages=1604–11.e4 |date=August 2013 |pmid=23582353 |pmc=3728272 |doi=10.1016/j.ophtha.2013.01.021 |url=}}</ref> |
|||
''Placeholder while looking for better citations'' |
|||
=== Alternative medicine === |
=== Alternative medicine === |
||
Revision as of 16:35, 18 March 2018
Tocopherols (/toʊˈkɒfəˌrɒl/;[1] TCP) are a class of organic chemical compounds (more precisely, various methylated phenols), many of which have vitamin E activity. Because the vitamin activity was first identified in 1936 from a dietary fertility factor in rats, it was given the name "tocopherol" from the Greek words "τόκος" [tókos, birth], and "φέρειν", [phérein, to bear or carry] meaning in sum "to carry a pregnancy," with the ending "-ol" signifying its status as a chemical alcohol.
α-Tocopherol is the main source found in supplements and in the European diet, where the main dietary sources are olive and sunflower oils,[2] while γ-tocopherol is the most common form in the American diet due to a higher intake of soybean and corn oil.[2][3]
Tocotrienols, which are related compounds, also have vitamin E activity. All of these various derivatives with vitamin activity may correctly be referred to as "vitamin E". Tocopherols and tocotrienols are fat-soluble antioxidants but also seem to have many other functions in the body.
Forms
Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes.
Both the tocopherols and tocotrienols occur in α (alpha), β (beta), γ (gamma) and δ (delta) forms, determined by the number and position of methyl groups on the chromanol ring.
| Form | Structure |
|---|---|
| alpha-Tocopherol | 
|
| beta-Tocopherol | 
|
| gamma-Tocopherol | 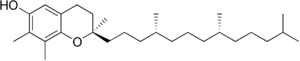
|
| delta-Tocopherol | 
|
The tocotrienols have the same methyl structure at the ring and the same Greek letter-methyl-notation, but differ from the analogous tocopherols by the presence of three double bonds in the hydrophobic side chain. The unsaturation of the tails gives tocotrienols only a single stereoisomeric carbon (and thus two possible isomers per structural formula, one of which occurs naturally), whereas tocopherols have 3 centers (and eight possible stereoisomers per structural formula, again, only one of which occurs naturally).
Each form has a different biological activity.[4][5] In general, the unnatural l-isomers of tocotrienols lack almost all vitamin activity, and half of the possible 8 isomers of the tocopherols (those with 2S chirality at the ring-tail junction) also lack vitamin activity. Of the stereoisomers which retain activity, increasing methylation, especially full methylation to the alpha-form, increases vitamin activity. In tocopherols, this is due to the preference of the tocopherol binding protein for the alpha-tocopherol form of the vitamin.
As a food additive, tocopherol is labeled with these E numbers: E306 (tocopherol), E307 (α-tocopherol), E308 (γ-tocopherol), and E309 (δ-tocopherol). These are all approved in the USA,[6] EU[7] and Australia and New Zealand[8] for use as antioxidants.
α-Tocopherol
Alpha-tocopherol is the form of vitamin E that is preferentially absorbed and accumulated in humans.[9] The measurement of "vitamin E" activity in international units (IU) was based on fertility enhancement by the prevention of miscarriages in pregnant rats relative to alpha-tocopherol.
Although the mono-methylated form ddd-gamma-tocopherol is the most prevalent form of vitamin E in oils, there is evidence that rats can methylate this form to the preferred alpha-tocopherol, since several generations of rats retained alpha-tocopherol tissue levels, even when fed only gamma-tocopherol through their lives.
There are three stereocenters in alpha-tocopherol, so this is a chiral molecule.[10] The eight stereoisomers of alpha-tocopherol differ in the arrangement of groups around these stereocenters. In the image of RRR-alpha-tocopherol below, all three stereocenters are in the R form. However, if the middle of the three stereocenters were changed (so the hydrogen was now pointing down and the methyl group pointing up), this would become the structure of RSR-alpha-tocopherol. These stereoisomers can also be named in an alternative older nomenclature, where the stereocenters are either in the d or l form.[11]

1 IU of tocopherol is defined as ⅔ milligrams of RRR-alpha-tocopherol (formerly named d-alpha-tocopherol or sometimes ddd-alpha-tocopherol). 1 IU is also defined as 1 milligram of an equal mix of the eight stereoisomers, which is a racemic mixture called all-rac-alpha-tocopheryl acetate. This mix of stereoisomers is often called dl-alpha-tocopheryl acetate, even though it is more precisely dl,dl,dl-alpha-tocopheryl acetate). However, 1 IU of this racemic mixture is not now considered equivalent to 1 IU of natural (RRR) α-tocopherol, and the Institute of Medicine and the USDA now convert IU's of the racemic mixture to milligrams of equivalent RRR using 1 IU racemic mixture = 0.45 "milligrams α-tocopherol".[12]
Tocotrienols
Tocotrienols, although less commonly known, also belong to the vitamin E family. Tocotrienols have four natural 2' d-isomers (they have a stereoisomeric carbon only at the 2' ring-tail position). The four tocotrienols (in order of decreasing methylation: d-alpha, d-beta, d-gamma, and d-delta-tocotrienol) have structures corresponding to the four tocopherols, except with an unsaturated bond in each of the three isoprene units that form the hydrocarbon tail, whereas tocopherols have a saturated phytyl tail (the phytyl tail of tocopherols gives the possibility for 2 more stereoisomeric sites in these molecules that tocotrienols do not have). Tocotrienol has been subject to fewer clinical studies and seen less research as compared to tocopherol. However, there is growing interest in the health effects of these compounds.[13]
Dietary recommendations
The U.S. Recommended Dietary Allowance (RDA) for adults is 15 mg/day. The RDA is based on the alpha-tocopherol form because it is the most active form as originally tested. However, they do caution individuals who consume low fat diets because vegetable oils are such a good dietary source of vitamin E. Vitamin E supplements are absorbed best when taken with meals.[14] The U.S. Institute of Medicine has set an upper tolerable intake level (UL) for vitamin E at 1,000 mg (1,500 IU) per day.[15] The European Food Safety Authority set UL of 300 mg alpha-tocopherol equivalents /day.[16]
α-Tocopherol equivalents
For dietary purposes, vitamin E activity of vitamin E isomers is expressed as α-tocopherol equivalents (a-TEs). One a-TE is defined by the biological activity of 1 mg (natural) d-alpha-Tocopherol in the resorption-gestation test. According to listings by FAO and others beta-tocopherol should be multiplied by 0.5, gamma-tocopherol by 0.1, and a-tocotrienol by 0.3.[4] The IU is converted to aTE by multiplying it with 0.67.[17] These factors do not correlate with the antioxidant activity of vitamin E isomers, where tocotrienols show even much higher activity in vivo.[18]
Sources
In general, food sources with the highest concentrations of vitamin E are vegetable oils, followed by nuts and seeds including whole grains. Adjusting for typical portion sizes, however, for many people in the United States the most important sources of vitamin E include commercial breakfast cereal and tomato sauce.[19] Although originally extracted from wheat germ oil, most natural vitamin E supplements are now derived from vegetable oils, usually soybean oil.
Vitamin E content per 100 g of source include:[20][21]
- Wheat germ oil (215.4 mg)
- Sunflower oil (55.8 mg)
- Almond oil (39.2 mg)
- Sunflower seed (35.17 mg)
- Almond (26.2 mg)
- Hazelnut (26.0 mg)
- Walnut oil (20.0 mg)
- Peanut oil (17.2 mg)
- Olive oil (12.0 mg)
- Poppyseed oil (11.4 mg)
- Peanut (9.0 mg)
- Pollard (2.4 mg)
- Maize (2.0 mg)
- Poppy seed (1.8 mg)
- Baru almonds (Dipteryx alata) (21.4 mg)
- Asparagus (1.5 mg)
- Oats (1.5 mg)
- Chestnut (1.2 mg)
- Coconut (1.0 mg)
- Tomatoes (0.9 mg)
- Walnut (0.7 mg)
- Carrots (0.6 mg)
- Goat's milk (0.1 mg)
A 100 g serving of certain fortified breakfast cereals may contain 24 mg (or more) vitamin E.[19]
The proportion of vitamin E to other tocopherols in a nutrient source varies greatly. For example, the tocopherol content is 96% vitamin E in almonds and 9% vitamin E in poppy seeds.[21]
Deficiency
Vitamin E deficiency is rare, and in almost all instances caused by an underlying disease rather than a diet low in vitamin E.[15] Vitamin E deficiency causes neurological problems due to poor nerve conduction. These include neuromuscular problems such as spinocerebellar ataxia and myopathies.[11] Deficiency can also cause anemia, due to oxidative damage to red blood cells.
Supplements
Commercial vitamin E supplements can be classified into several distinct categories:
- Fully synthetic vitamin E, "dl-alpha-tocopherol", the most inexpensive, most commonly sold supplement form usually as the acetate ester.
- Semi-synthetic "natural source" vitamin E esters, the "natural source" forms used in tablets and multiple vitamins. These are highly fractionated d-alpha tocopherol or its esters, often made by synthetic methylation of gamma and beta d,d,d tocopherol vitamers extracted from plant oils.
- Less fractionated "natural mixed tocopherols" and high d-gamma-tocopherol fraction supplements.
Synthetic all-racemic
Synthetic vitamin E derived from petroleum products is manufactured as all-racemic alpha tocopheryl acetate with a mixture of eight stereoisomers. In this mixture, one alpha-tocopherol molecule in eight molecules are in the form of RRR-alpha-tocopherol (12.5% of the total).[22]
The 8-isomer all-rac vitamin E is always marked on labels simply as dl-tocopherol or dl-tocopheryl acetate, even though it is (if fully written out) actually dl,dl,dl-tocopherol. The present largest manufacturers of this type are DSM and BASF.
(An earlier semisynthetic vitamin E actually contained 50% d,d,d-alpha tocopherol moiety and 50% l,d,d-alpha-tocopherol moiety, as synthesized by an earlier process which started with a plant sterol intermediate with the correct chirality in the tail, and thus resulted in a racemic mixture at only one chiral center. This form, known as 2-ambo tocopherol, is no longer made.)
Natural alpha-tocopherol is the RRR-alpha (or ddd-alpha) form. The synthetic dl,dl,dl-alpha ("dl-alpha") form is not as active as the natural ddd-alpha ("d-alpha") tocopherol form. This is mainly due to reduced vitamin activity of the 4 possible stereoisomers which are represented by the l or S enantiomer at the first stereocenter (an S or l configuration between the chromanol ring and the tail, i.e., the SRR, SRS, SSR, and SSS stereoisomers).[10] The 3 unnatural "2R" stereoisomers with natural R configuration at this 2' stereocenter, but S at one of the other centers in the tail (i.e., RSR, RRS, RSS), appear to retain substantial RRR vitamin activity, because they are recognized by the alpha-tocopherol transport protein, and thus maintained in the plasma, where the other four stereoisomers (SRR, SRS, SSR, and SSS) are not. Thus, the synthetic all-rac-α-tocopherol in theory would have approximately half the vitamin activity of RRR-alpha-tocopherol in humans. Experimentally, the ratio of activities of the 8 stereoisomer racemic mixture to the natural vitamin, is 1 to 1.36 in the rat pregnancy model (suggesting a measured activity ratio of 1/1.36 = 74% of natural, for the 8-isomer racemic mix).[23]
Although it is clear that mixtures of stereoisomers are not as active as the natural RRR-alpha-tocopherol form, in the ratios discussed above, specific information on any side effects of the seven synthetic vitamin E stereoisomers is not readily available.
Esters

Manufacturers also commonly convert the phenol form of the vitamins (with a free hydroxyl group) to esters, using acetic or succinic acid. These tocopheryl esters are more stable and are easy to use in vitamin supplements. Alpha tocopheryl esters are de-esterified in the gut and then absorbed as the free tocopherol.[24][25] Tocopheryl nicotinate and tocopheryl linolate esters are also used in cosmetics and some pharmaceuticals.
Mixed tocopherols
"Mixed tocopherols" in the US contain at least 20% w/w other natural R, R,R- tocopherols, i.e. R, R,R-alpha-tocopherol content plus at least 25% R, R,R-beta-, R, R,R-gamma-, R, R,R-delta-tocopherols.[citation needed]
Some brands may contain 200% w/w or more of the other tocopherols and measurable tocotrienols. Some mixed tocopherols with higher gamma-tocopherol content are marketed as "High Gamma-Tocopherol." The label should report each component in milligrams, except R, R,R-alpha-tocopherol may still be reported in IU. Mixed tocopherols can also be found in other nutritional supplements.[citation needed]
Pharmacology
A Cochrane review published in 2017 on antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration (AMD) identified only one vitamin E clinical trial. That trial compared 500 IU/day of alpha-tocopherol to placebo for four years and reported no effect on the progression of age-related macular degeneration in people already diagnosed with the condition.[26] Another Cochrane review, same year, same authors, reviewed the literature on alpha-tocopherol preventing the development of age-related macular degeneration. This review identified four trials, duration 4-10 years, and reported no change to risk of developing AMD.[27] A large clinical trial known as AREDS compared beta-carotene (15 mg), vitamin C (500 mg) and alpha-tocopherol (400 IU) to placebo for up to 10 years, with a conclusion that the anti-oxidant combination significantly slowed the progression of AMD. However, because there was no group in the trial receiving only vitamin E, no conclusions could be drawn as to the contribution of the vitamin to the effect.[28]
Alternative medicine
Excessive intake of vitamin E may increase risk of bleeding, and a 2005 meta-analysis found that high-dosage vitamin E supplements may increase all-cause mortality.[29] A Cochrane review of 2007 also found an increase in mortality, of 4% (Relative Risk 1.04, 95% confidence interval 1.01-1.07).[30]
Proponents of megavitamin, orthomolecular, and naturally based therapies have for the last two thirds of a century advocated and used the natural tocopherols, often mixed tocopherols with an additional 25%-200% w/w d-beta-, d-gamma-, and d-delta-tocopherol.[3][31] Studies on vitamin E have largely concentrated on use of either a synthetic all-racemic ("d, l-") alpha tocopheryl ester (acetate or succinate) or a semi-synthetic d-alpha tocopheryl ester (acetate or succinate).
Alzheimer's disease
Alzheimer's disease is a wasting disease of the brain. As oxidative stress may be involved in the pathogenesis of Alzheimer's, tocopherols have been tested as a means of both prevention and treatment. The results of these studies have been mixed, with some research suggesting that high levels of vitamin E in the diet may reduce the risk of Alzheimer's whilst other studies found no such link.[32] Studies on progression have also been contradictory, with the Alzheimer's Disease Cooperative Study suggesting that vitamin E supplementation may be beneficial but a later trial finding no clinical benefit.[33] Owing to this contradictory and confusing evidence, vitamin E or tocopherol supplements are not currently recommended for treating or preventing Alzheimer's disease.[34]
Cancer
As of 2009[update], human trials and surveys that have investigated potential association of vitamin E intake with incidence of cancer remain generally inconclusive.
Some evidence associates higher intake of vitamin E with a decreased incidence of prostate cancer (see ATBC study) and breast cancer. Some studies correlate additional cofactors, such as specific vitamin E isomers, e.g. gamma-tocopherol, and other nutrients, e.g. selenium, with dramatic risk reductions in prostate cancer.[35] However an examination of the effect of dietary factors, including vitamin E, on incidence of postmenopausal breast cancer in more than 18,000 women from New York State did not associate a greater vitamin E intake with a reduced risk of developing breast cancer. A study of the effect on lung cancer in smokers also showed no benefit.[36]
Recent studies have found that increased intake of vitamin E, especially among smokers, may be responsible for an increase in the incidence of lung cancer, with one study finding an increase in the incidence of lung cancer of 7% for each 100 IU of vitamin E taken daily.[37][38][39]
A potential confounding factor is the form of Vitamin E used in these studies. As explained earlier, synthetic, racemic mixtures of Vitamin E isomers are not bioequivalent to natural, non-racemic mixtures yet are widely used academically and commercially.[40] The SELECT study for prostate cancer used racemic alpha-tocopherol, for instance, and has shown no benefit.[41] The study, cited above, showing a modest increase in cancer risk with Vitamin E supplementation, reported that more than 90% of its respondents used a racemic form of Vitamin E (d,l-alpha-tocopherol).[42] A meta-analysis of studies using Vitamin E, sorting results by the form (racemic vs non-racemic) used, is necessary.
Cataracts
Antioxidants are being studied to determine whether they can help prevent or delay age-related growth of cataracts, a clouding of the tissue of the lens of the eye. A controlled trial of high doses of vitamins C and E and beta carotene found no effect on the risk of developing cataracts.[43] Similarly, a trial using vitamin E alone found that vitamin E supplementation produced no change in the risk of developing cataracts or the rate of progression of existing cataracts.[44]
Glaucoma
A 2007 study published in the European Journal of Ophthalmology found that, along with other treatments for glaucoma, adding alpha-tocopherol appeared to help protect the retina from glaucomatous damage. Groups receiving 300 mg and 600 mg per day of alpha-tocopherol, delivered orally, showed statistically significant decreases in the resistivity index in the posterior ciliary arteries and in the pulsatility index in the ophthalmic arteries after six and twelve months of therapy. Alpha-tocopherol-treated patients also had significantly lower differences in mean visual field deviations."[45]
Heart disease
Preliminary research has led to a widely held belief that vitamin E may help prevent or delay coronary heart disease, but larger controlled studies have not shown any benefit.[46] One potential explanation could be it is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes.[47] Many researchers advance the belief that oxidative modification of LDL-cholesterol (sometimes called "bad" cholesterol) promotes blockages in coronary arteries that may lead to atherosclerosis and heart attacks.[citation needed] Vitamin E may help prevent or delay coronary heart disease by limiting the oxidation of LDL-cholesterol. Vitamin E also may help prevent the formation of blood clots, which could lead to a heart attack. Observational studies have associated lower rates of heart disease with higher vitamin E intake. A study of approximately 90,000 nurses suggested that the incidence of heart disease was 30% to 40% lower among nurses with the highest intake of vitamin E from diet and supplements. The range of intakes from both diet and supplements in this group was 21.6 to 1,000 IU (32 to 1,500 mg), with the median intake being 208 IU (139 mg). A 1994 review of 5,133 Finnish men and women aged 30 – 69 years suggested that increased dietary intake of vitamin E was associated with decreased mortality (death) from heart disease.
Despite these promising observations, randomized clinical trials have consistently shown lack of benefit to the role of vitamin E supplements in heart disease. The Heart Outcomes Prevention Evaluation (HOPE) Study followed almost 10,000 patients for 4.5 years who were at high risk for heart attack or stroke. In this intervention study the subjects who received 265 mg (400) IU of vitamin E daily did not experience significantly fewer cardiovascular events or hospitalizations for heart failure or chest pain when compared to those who received a placebo. The researchers suggested that it is unlikely that the vitamin E supplement provided any protection against cardiovascular disease in the HOPE study. This study is continuing, to determine whether a longer duration of intervention with vitamin E supplements will provide any protection against cardiovascular disease.
Furthermore, meta analysis of several trials of antioxidants, including vitamin E, have not shown any benefit to vitamin E supplementation for preventing coronary heart disease.[48] One study suggested that Vitamin E (as alpha-tocopherol only) supplementation may increase the risk for heart failure.[49] Supplementing alpha-tocopherol without gamma-tocopherol is known to lead to reduced serum gamma- and delta-tocopherol concentrations.[50]
A large-scale 10-year study published in 2007 examined the rates of venous thromboembolism (VTE) and pulmonary embolism in women taking 600 IU of vitamin E on alternate days. The study found a significant reduction in VTE especially in women who had a history of thrombtic events or a genetic predispostion.[51]
Parkinson's disease
In May 2005, The Lancet Neurology published a study suggesting that vitamin E may help protect against Parkinson's disease.[52] Individuals with moderate to high intakes of dietary vitamin E were found to have a lower risk of Parkinson's. No conclusion could be made whether supplemental vitamin E has the same effect.[53] Other trials have tested whether giving vitamin E supplements reduces the risk of Parkinson's disease, or if they can slow the progression of the disease. In a 1998 study, vitamin E supplements had no effect on the rate of progression.[54]
Pregnancy
Recent studies into the use of both vitamin C and the single isomer vitamin E esters as possible aids in preventing oxidative stress leading to pre-eclampsia has failed to show significant benefits,[55] but did increase the rate of babies born with a low birthweight in one study.[56]
Preservative
Tocopherols are sometimes used as a food preservative to prevent oils from going rancid and in liquid castile soap made from coconut, olive, jojoba or hemp oil.
Topical use
Vitamin E is widely used as an inexpensive antioxidant in cosmetics and foods. Vitamin E containing products are commonly used in the belief that vitamin E is good for the skin; many cosmetics include it, often labeled as tocopherol acetate, tocopheryl linoleate or tocopheryl nicotinate. Some individuals experience allergic reactions to some tocopheryl esters or develop a rash and hives that may spread over the entire body from the use of topical products with alpha tocopheryl esters.[57]
Vitamin E is often claimed by manufacturers of skin creams and lotions to play a role in encouraging skin healing and reducing scarring after injuries such as burns on the basis of limited research,[58] but the weak evidence of a benefit of silicon gel sheeting with or without added Vitamin E is limited by the poor quality of the research.[59] A 2015 review concluded that there is insufficient evidence to support claims for healing burns or other types of wounds.[60]
History
During feeding experiments with rats Herbert McLean Evans concluded in 1922 that besides vitamins B and C, an unknown vitamin existed.[61] Although every other nutrition was present, the rats were not fertile. This condition could be changed by additional feeding with wheat germ. It took several years until 1936 when the substance was isolated from wheat germ and the formula C29H50O2 was determined. Evans also found that the compound reacted like an alcohol and concluded that one of the oxygen atoms was part of an OH (hydroxyl) group. As noted in the introduction, the vitamin was given its name by Evans from Greek words meaning "to bear young" with the addition of the -ol as an alcohol.[62] The structure was determined shortly thereafter in 1938.[63]
See also
References
- ^ "Tocopherol". Dictionary.com Unabridged (Online). n.d. Retrieved 28 February 2018.
- ^ a b Wagner, Karl-Heinz; Afaf Kamal-Eldin; Ibrahim Elmadfa (2004). "Gamma-tocopherol--an underestimated vitamin?". Annals of nutrition and metabolism. 48 (3): 169–88. doi:10.1159/000079555. PMID 15256801.
In North America, the intake of γ-tocopherol has been estimated to exceed that of α-tocopherol by a factor of 2–4 ... due to the fact that soybean oil is the predominant vegetable oil in the American diet (76.4%) followed by corn oil and canola oil (both 7%) ... The supply of dietary fats ... is much more diverse in Europe ... The oils mainly consumed in Europe, i.e. sunflower, olive and canola oil, provide less γ-tocopherol but more α-tocopherol ... [T]he ratio of α-:γ-tocopherol is at least 1:2. Therefore, the average γ-tocopherol intake can be estimated as 4–6 mg/day, which is about 25–35% of the USA intake. In accordance with the lower estimated European intake of γ-tocopherol, the serum levels of γ-tocopherol in European populations are 4–20 times lower than that of α-tocopherol
- ^ a b Jiang, Q; Christen, S; Shigenaga, MK; Ames, BN (2001). "gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention". The American Journal of Clinical Nutrition. 74 (6): 714–22. PMID 11722951.
- ^ a b http://www.fao.org/docrep/004/Y2809E/y2809e0f.htm Summary of the role of vitamin E in human metabolic processes
- ^ Burton, G. W.; Ingold, K. U. (1981). "Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro". J. Am. Chem. Soc. 103: 6472–6477. doi:10.1021/ja00411a035.
- ^ US Food and Drug Administration: "Listing of Food Additives Status Part II". Archived from the original on November 8, 2011. Retrieved 2011-10-27.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ^ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ^ Rigotti, A (2007). "Absorption, transport, and tissue delivery of vitamin E". Molecular Aspects of Medicine. 28 (5–6): 423–36. doi:10.1016/j.mam.2007.01.002. PMID 17320165.
- ^ a b Jensen, S; Lauridsen, C (2007). "α‐Tocopherol Stereoisomers". Vitamins & Hormones. 76: 281–308. doi:10.1016/S0083-6729(07)76010-7. PMID 17628178.
- ^ a b Brigelius-Flohé R, Traber MG (1 July 1999). "Vitamin E: function and metabolism". FASEB J. 13 (10): 1145–55. doi:10.1096/fasebj.13.10.1145. PMID 10385606.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference, Release 20 USDA, February 2008
- ^ Sen, C; Khanna, S; Roy, S (2006). "Tocotrienols: Vitamin E beyond tocopherols". Life Sciences. 78 (18): 2088–98. doi:10.1016/j.lfs.2005.12.001. PMC 1790869. PMID 16458936.
- ^ Iuliano, L.; Micheletta, F.; Maranghi, M.; Frati, G.; Diczfalusy, U.; Violi, F. (2001). "Bioavailability of Vitamin E as Function of Food Intake in Healthy Subjects: Effects on Plasma Peroxide-Scavenging Activity and Cholesterol-Oxidation Products". Arteriosclerosis, Thrombosis, and Vascular Biology. 21 (10): e34–e37. doi:10.1161/hq1001.098465.
- ^ a b Vitamin E Fact sheet National Institutes of Health, Office of Dietary Supplements
- ^ Opinion on mixed tocopherols, tocotrienol tocopherol and tocotrienols as sources for vitamin E added as a nutritional substance in food supplements The EFSA Journal (2008) 640,1-34.
- ^ http://www.ncc.umn.edu/products/databaseNUTvitamins.html University of Minnesota Nutrition Coordinating Center on Vitamins
- ^ Packer L, Weber SU, Rimbach G (February 2001). "Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling". J. Nutr. 131 (2): 369S–73S. PMID 11160563.
- ^ a b http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/SR22/nutrlist/sr22w323.pdf
- ^ J. Bauernfeind in: L. J. Machlin (ed.): Vitamin E – A Comprehensive Treatise, Marcel Dekker, New York 1980, p. 99
- ^ a b "USDA National Nutrient Database for Standard Reference". USDA Agricultural Research Service. Retrieved 2009-11-26.
- ^ Weiser H, Riss G, Kormann AW (1 October 1996). "Biodiscrimination of the eight alpha-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats". J. Nutr. 126 (10): 2539–49. PMID 8857515.
- ^ "Taken together, these data indicate that of the eight stereoisomers (RRR, RSR, RRS, RSS, SRR, SSR, SRS, SSS) in all-rac-α-tocopherol, only the four 2R-forms (RRR, RSR, RSS, RRS) are recognized by α-TTP and maintained in the plasma. Indeed, the Food and Nutrition Board (Food and Nutrition Board and Institute of Medicine, 2000) has defined that only α-tocopherol, specifically the 2R-forms of α-tocopherol, can fulfill the human requirement for vitamin E. Thus, all-rac-α-tocopherol has only half the activity of RRR-α-tocopherol." Taken from the discussion in Lauridsen, C.; Engel, H.; Craig, AM.; Traber, MG. (Mar 2002). "Relative bioactivity of dietary RRR- and all-rac-alpha-tocopheryl acetates in swine assessed with deuterium-labeled vitamin E" (PDF). J Anim Sci. 80 (3): 702–7. PMID 11890405.
- ^ Mathias PM, Harries JT, Peters TJ, Muller DP (July 1981). "Studies on the in vivo absorption of micellar solutions of tocopherol and tocopheryl acetate in the rat: demonstration and partial characterization of a mucosal esterase localized to the endoplasmic reticulum of the enterocyte". J. Lipid Res. 22 (5): 829–37. PMID 7288289.
- ^ Ajandouz el H, Castan S, Jakob S, Puigserver A (2006). "A fast, sensitive HPLC method for the determination of esterase activity on alpha-tocopheryl acetate". J Chromatogr Sci. 44 (10): 631–3. doi:10.1093/chromsci/44.10.631. PMID 17254374.
- ^ Evans JR, Lawrenson JG (July 2017). "Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration". Cochrane Database Syst Rev. 7: CD000254. doi:10.1002/14651858.CD000254.pub4. PMID 28756618.
- ^ Evans JR, Lawrenson JG (July 2017). "Antioxidant vitamin and mineral supplements for preventing age-related macular degeneration". Cochrane Database Syst Rev. 7: CD000253. doi:10.1002/14651858.CD000253.pub4. PMID 28756617.
- ^ Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD (August 2013). "Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35". Ophthalmology. 120 (8): 1604–11.e4. doi:10.1016/j.ophtha.2013.01.021. PMC 3728272. PMID 23582353.
- ^ Robertsii, L; Oates, J; Linton, M; Fazio, S; Meador, B; Gross, M; Shyr, Y; Morrow, J (2007). "The relationship between dose of vitamin E and suppression of oxidative stress in humans". Free Radical Biology and Medicine. 43 (10): 37–46. doi:10.1016/j.freeradbiomed.2007.06.019. PMC 2072864. PMID 17936185.
- ^ Bjelakovic, G; Nikolova, D; Gluud, LL; Simonetti, RG; Gluud, C (2007). "Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis". JAMA: The Journal of the American Medical Association. 297 (8): 842–57. doi:10.1001/jama.297.8.842. PMID 17327526.
- ^ Gaziano, JM (2004). "Vitamin E and cardiovascular disease: observational studies". Annals of the New York Academy of Sciences. 1031: 280–91. doi:10.1196/annals.1331.028. PMID 15753154.
- ^ Frank, Bradford; Gupta, Sanjay (2005). "A Review of Antioxidants and Alzheimer's Disease". Annals of Clinical Psychiatry. 17 (4): 269–86. doi:10.1080/10401230500296428. PMID 16402761.
- ^ Ricciarelli, R; Argellati, F; Pronzato, M; Domenicotti, C (2007). "Vitamin E and neurodegenerative diseases". Molecular Aspects of Medicine. 28 (5–6): 591–606. doi:10.1016/j.mam.2007.01.004. PMID 17306357.
- ^ Boothby, L. A; Doering, PL (2005). "Vitamin C and Vitamin E for Alzheimer's Disease". Annals of Pharmacotherapy. 39 (12): 2073–80. doi:10.1345/aph.1E495. PMID 16227450.
- ^ Helzlsouer, K. J.; Huang, HY; Alberg, AJ; Hoffman, S; Burke, A; Norkus, EP; Morris, JS; Comstock, GW (2000). "Association Between alpha-Tocopherol, gamma-Tocopherol, Selenium, and Subsequent Prostate Cancer". Journal of the National Cancer Institute. 92 (24): 2018–23. doi:10.1093/jnci/92.24.2018. PMID 11121464.
- ^ "The Effect of Vitamin E and Beta Carotene on the Incidence of Lung Cancer and Other Cancers in Male Smokers". New England Journal of Medicine. 330 (15): 1029–35. 1994. doi:10.1056/NEJM199404143301501. PMID 8127329.
- ^ Cancer Research UK: Jury still out over vitamin E supplements and increased lung cancer risk
- ^ Nursing in Practice - Vitamin E "linked to cancer"
- ^ eCanadaNow.com - Vitamin E Linked To Increased Risk of Lung Cancer
- ^ Jensen, SK; Lauridsen, C (2007). "Alpha-tocopherol stereoisomers". Vitamins and hormones. Vitamins & Hormones. 76: 281–308. doi:10.1016/S0083-6729(07)76010-7. ISBN 9780123735928. PMID 17628178.
- ^ Klein, EA (2004). "Selenium and vitamin E cancer prevention trial". Annals of the New York Academy of Sciences. 1031: 234–41. doi:10.1196/annals.1331.023. PMID 15753149.
- ^ Slatore, C. G.; Littman, A. J.; Au, D. H.; Satia, J. A.; White, E. (2007). "Long-Term Use of Supplemental Multivitamins, Vitamin C, Vitamin E, and Folate Does Not Reduce the Risk of Lung Cancer". American Journal of Respiratory and Critical Care Medicine. 177 (5): 524–530. doi:10.1164/rccm.200709-1398OC. PMC 2258445. PMID 17989343.
- ^ Age-Related Eye Disease Study Research Group (2001). "A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9". Archives of ophthalmology. 119 (10): 1439–52. doi:10.1001/archopht.119.10.1439. PMC 1472812. PMID 11594943.
- ^ McNeil, J; Robman, L; Tikellis, G; Sinclair, MI; McCarty, CA; Taylor, HR (2004). "Vitamin E supplementation and cataract*1Randomized controlled trial". Ophthalmology. 111 (1): 75–84. doi:10.1016/j.ophtha.2003.04.009. PMID 14711717.
- ^ Engin, KN; Engin, G; Kucuksahin, H; Oncu, M; Engin, G; Guvener, B (2007). "Clinical evaluation of the neuroprotective effect of alpha-tocopherol against glaucomatous damage". European Journal of Ophthalmology. 17 (4): 528–33. PMID 17671926.
- ^ Sesso, H. D.; Buring, J. E.; Christen, W. G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J. E.; et al. (2008). "Vitamins E and C in the Prevention of Cardiovascular Disease in Men: The Physicians' Health Study II Randomized Controlled Trial". JAMA: The Journal of the American Medical Association. 300 (18): 2123–2133. doi:10.1001/jama.2008.600. PMC 2586922. PMID 18997197.
- ^ Raghavamenon, A.; Garelnabi, M.; Babu, S.; Aldrich, A.; Litvinov, D.; Parthasarathy, S. (2009). "α-Tocopherol Is Ineffective in Preventing the Decomposition of Preformed Lipid Peroxides and May Promote the Accumulation of Toxic Aldehydes: A Potential Explanation for the Failure of Antioxidants to Affect Human Atherosclerosis". Antioxidants & Redox Signaling. 11 (6): 1237–1248. doi:10.1089/ars.2008.2248. PMC 2842134. PMID 19186999.
- ^ Vivekananthan, D; Penn, M; Sapp, S; Hsu, A; Topol, E (2003). "Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials". The Lancet. 361 (9374): 2017–23. doi:10.1016/S0140-6736(03)13637-9. PMID 12814711.
- ^ Lonn, E; Bosch, J; Yusuf, S; Sheridan, P; Pogue, J; Arnold, JM; Ross, C; Arnold, A; et al. (2005). "Effects of Long-term Vitamin E Supplementation on Cardiovascular Events and Cancer: A Randomized Controlled Trial". JAMA: The Journal of the American Medical Association. 293 (11): 1338–47. doi:10.1001/jama.293.11.1338. PMID 15769967.
- ^ Huang, HY; Appel, LJ (2003). "Supplementation of diets with alpha-tocopherol reduces serum concentrations of gamma- and delta-tocopherol in humans". The Journal of Nutrition. 133 (10): 3137–40. PMID 14519797.
- ^ Glynn, R. J.; Ridker, P. M; Goldhaber, S. Z.; Zee, R. Y.L.; Buring, J. E. (2007). "Effects of Random Allocation to Vitamin E Supplementation on the Occurrence of Venous Thromboembolism: Report From the Women's Health Study". Circulation. 116 (13): 1497–1503. doi:10.1161/CIRCULATIONAHA.107.716407. PMID 17846285.
- ^ Etminan, Mahyar; Gill, Sudeep S; Samii, Ali (2005). "Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis". The Lancet Neurology. 4 (6): 362–5. doi:10.1016/S1474-4422(05)70097-1.
- ^ "Vitamin E cuts Parkinson's risk". BBC News. 19 May 2005. Retrieved 2008-01-01.
- ^ Shoulson I (September 1998). "DATATOP: a decade of neuroprotective inquiry. Parkinson Study Group. Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism". Annals of Neurology. 44 (3 Suppl 1): S160–6. doi:10.1002/ana.410440724. PMID 9749589.
- ^ Rumbold, AR; Crowther, CA; Haslam, RR; Dekker, GA; Robinson, JS; Acts Study, Group (2006). "Vitamins C and E and the risks of preeclampsia and perinatal complications". The New England Journal of Medicine. 354 (17): 1796–806. doi:10.1056/NEJMoa054186. PMID 16641396.
- ^ Poston, L; Briley, AL; Seed, PT; Kelly, FJ; Shennan, AH (2006). "Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial". The Lancet. 367 (9517): 1145–54. doi:10.1016/S0140-6736(06)68433-X. PMID 16616557.
- ^ Topical Vitamin E formulations: Not always benign, Skin Therapy Letter Vol. 1, No. 3, January 1996
- ^ Palmieri, Beniamino; Gozzi, Glauco; Palmieri, Gaspare (1995). "Vitamine E added silicone gel sheets for treatment of hypertrophic scars and keloids". International Journal of Dermatology. 34 (7): 506–9. doi:10.1111/j.1365-4362.1995.tb00628.x. PMID 7591421.
- ^ O'Brien, Lisa; Jones, Daniel J. (2013-09-12). "Silicone gel sheeting for preventing and treating hypertrophic and keloid scars". The Cochrane Database of Systematic Reviews (9): CD003826. doi:10.1002/14651858.CD003826.pub3. ISSN 1469-493X. PMID 24030657.
- ^ Sidgwick GP, McGeorge D, Bayat A (2015). "A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring". Arch. Dermatol. Res. 307 (6): 461–77. doi:10.1007/s00403-015-1572-0. PMC 4506744. PMID 26044054.
- ^ Evans, H. M.; Bishop, K. S. (1922). "On the Existence of a Hitherto Unrecognized Dietary Factor Essential for Reproduction". Science. 56 (1458): 650–651. doi:10.1126/science.56.1458.650. PMID 17838496.
- ^ Evans H. M.; Emerson O. H.; Emerson G. A. (1 February 1936). "The isolation from wheat germ oil of an alcohol, a-tocopherol, having the properties of vitamin E". Journal of Biological Chemistry. 113 (1): 319–332.
- ^ Fernholz, E. (1938). "On the Constitution of α-Tocopherol". Journal of the American Chemical Society. 60 (3): 700–705. doi:10.1021/ja01270a057.
External links
- US Office of Dietary Supplements article on Vitamin E
- Vitamin E risk assessment, Expert Group on Vitamins and Minerals, UK Food Standards Agency, 2003
