Cobalt: Difference between revisions
m Reverted edits by 184.179.123.247 (talk) to last revision by Anbu121 (HG) |
|||
| Line 28: | Line 28: | ||
Cobalt is a weakly reducing metal that is protected from oxidation by a [[Passivation (chemistry)|passivating]] oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces [[Cobalt(II,III) oxide|Co<sub>3</sub>O<sub>4</sub>]] which loses oxygen at 900 °C to give the [[Cobalt(II) oxide|monoxide]] CoO.<ref name="HollemanAF"/> The metal reacts with F<sub>2</sub> at 520K to give [[Cobalt(III) fluoride|CoF<sub>3</sub>]], with Cl<sub>2</sub>, Br<sub>2</sub> and I<sub>2</sub>, the corresponding binary halides were formed. It has no reaction with [[hydrogen|H<sub>2</sub>]] and [[nitrogen|N<sub>2</sub>]] even when heated, but do react with [[boron]], [[carbon]], [[phosphorus]], [[arsenic]] and [[sulphur]].<ref>{{Housecroft3rd|page=722}}</ref> |
Cobalt is a weakly reducing metal that is protected from oxidation by a [[Passivation (chemistry)|passivating]] oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces [[Cobalt(II,III) oxide|Co<sub>3</sub>O<sub>4</sub>]] which loses oxygen at 900 °C to give the [[Cobalt(II) oxide|monoxide]] CoO.<ref name="HollemanAF"/> The metal reacts with F<sub>2</sub> at 520K to give [[Cobalt(III) fluoride|CoF<sub>3</sub>]], with Cl<sub>2</sub>, Br<sub>2</sub> and I<sub>2</sub>, the corresponding binary halides were formed. It has no reaction with [[hydrogen|H<sub>2</sub>]] and [[nitrogen|N<sub>2</sub>]] even when heated, but do react with [[boron]], [[carbon]], [[phosphorus]], [[arsenic]] and [[sulphur]].<ref>{{Housecroft3rd|page=722}}</ref> |
||
==Compounds== |
==Compounds are gay== |
||
{{Category see also|Cobalt compounds}} |
{{Category see also|Cobalt compounds}} |
||
Common [[oxidation states]] of cobalt include +2 and +3, although compounds with oxidation states ranging from −3 to +4 are also known. A common oxidation state for simple compounds is +2. Cobalt(II) salts form the red-pink [Co(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup> complex in aqueous solution. Addition of chloride gives the intensely blue {{chem|[CoCl|4|]|2-}}.<ref name=greenwood/><!--Cobalt compounds release a blue-green flame when heated. I could not find a good reference for it.--> |
Common [[oxidation states]] of cobalt include +2 and +3, although compounds with oxidation states ranging from −3 to +4 are also known. A common oxidation state for simple compounds is +2. Cobalt(II) salts form the red-pink [Co(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup> complex in aqueous solution. Addition of chloride gives the intensely blue {{chem|[CoCl|4|]|2-}}.<ref name=greenwood/><!--Cobalt compounds release a blue-green flame when heated. I could not find a good reference for it.--> |
||
===Oxygen and chalcogen compounds |
===Oxygen and chalcogen compounds are gay== |
||
Several [[oxide]]s of cobalt are known. Green [[cobalt(II) oxide]] (CoO) has [[Cubic crystal system|rocksalt]] structure. It is readily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)<sub>3</sub>). At temperatures of 600–700 °C, CoO oxidizes to the blue [[cobalt(II,III) oxide]] (Co<sub>3</sub>O<sub>4</sub>), which has a [[spinel]] structure.<ref name=greenwood>{{Greenwood&Earnshaw2nd|pages=1117–1119}}</ref> Black [[cobalt(III) oxide]] (Co<sub>2</sub>O<sub>3</sub>) is also known.<ref>{{cite book|page=107|title=The history and use of our earth's chemical elements: a reference guide|author=Krebs, Robert E.|edition=2|publisher=Greenwood Publishing Group|year=2006|isbn=0-313-33438-2}}</ref> Cobalt oxides are [[antiferromagnetic]] at low [[temperature]]: CoO ([[Neel temperature]] 291 K) and Co<sub>3</sub>O<sub>4</sub> (Neel temperature: 40 K), which is analogous to [[magnetite]] (Fe<sub>3</sub>O<sub>4</sub>), with a mixture of +2 and +3 oxidation states.<ref>{{cite journal|last1=Petitto|first1=Sarah C.|last2=Marsh|first2=Erin M.|last3=Carson|first3=Gregory A.|last4=Langell|first4=Marjorie A.|title=Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water|url=http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1021&context=chemistrylangell|journal=Journal of Molecular Catalysis A: Chemical|volume=281|pages=49|year=2008|doi=10.1016/j.molcata.2007.08.023}}</ref> |
Several [[oxide]]s of cobalt are known. Green [[cobalt(II) oxide]] (CoO) has [[Cubic crystal system|rocksalt]] structure. It is readily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)<sub>3</sub>). At temperatures of 600–700 °C, CoO oxidizes to the blue [[cobalt(II,III) oxide]] (Co<sub>3</sub>O<sub>4</sub>), which has a [[spinel]] structure.<ref name=greenwood>{{Greenwood&Earnshaw2nd|pages=1117–1119}}</ref> Black [[cobalt(III) oxide]] (Co<sub>2</sub>O<sub>3</sub>) is also known.<ref>{{cite book|page=107|title=The history and use of our earth's chemical elements: a reference guide|author=Krebs, Robert E.|edition=2|publisher=Greenwood Publishing Group|year=2006|isbn=0-313-33438-2}}</ref> Cobalt oxides are [[antiferromagnetic]] at low [[temperature]]: CoO ([[Neel temperature]] 291 K) and Co<sub>3</sub>O<sub>4</sub> (Neel temperature: 40 K), which is analogous to [[magnetite]] (Fe<sub>3</sub>O<sub>4</sub>), with a mixture of +2 and +3 oxidation states.<ref>{{cite journal|last1=Petitto|first1=Sarah C.|last2=Marsh|first2=Erin M.|last3=Carson|first3=Gregory A.|last4=Langell|first4=Marjorie A.|title=Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water|url=http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1021&context=chemistrylangell|journal=Journal of Molecular Catalysis A: Chemical|volume=281|pages=49|year=2008|doi=10.1016/j.molcata.2007.08.023}}</ref> |
||
The principal [[chalcogen]]ides of cobalt include the black [[cobalt(II) sulfide]]s, CoS<sub>2</sub>, which adopts a [[pyrite]]-like structure, and [[cobalt(III) sulfide|Co<sub>2</sub>S<sub>3</sub>]]. [[Pentlandite]] (Co<sub>9</sub>S<sub>8</sub>) is metal-rich.<ref name=greenwood/> |
The principal [[chalcogen]]ides of cobalt include the black [[cobalt(II) sulfide]]s, CoS<sub>2</sub>, which adopts a [[pyrite]]-like structure, and [[cobalt(III) sulfide|Co<sub>2</sub>S<sub>3</sub>]]. [[Pentlandite]] (Co<sub>9</sub>S<sub>8</sub>) is metal-rich.<ref name=greenwood/> |
||
===Halides |
===Halides are gay== |
||
[[File:Cobalt(II)-chloride-hexahydrate-sample.jpg|thumb|left| Cobalt(II) chloride hexahydrate]] |
[[File:Cobalt(II)-chloride-hexahydrate-sample.jpg|thumb|left| Cobalt(II) chloride hexahydrate]] |
||
Four dihalides of cobalt(II) are known: [[cobalt(II) fluoride]] (CoF<sub>2</sub>, pink), [[cobalt(II) chloride]] (CoCl<sub>2</sub>, blue), [[cobalt(II) bromide]] (CoBr<sub>2</sub>, green), [[cobalt(II) iodide]] (CoI<sub>2</sub>, blue-black). These halides exist in anhydrous and hydrated forms. Whereas the anhydrous dichloride is blue, the hydrate is red.<ref name=greenwood2>{{Greenwood&Earnshaw2nd|pages=1119–1120}}</ref> |
Four dihalides of cobalt(II) are known: [[cobalt(II) fluoride]] (CoF<sub>2</sub>, pink), [[cobalt(II) chloride]] (CoCl<sub>2</sub>, blue), [[cobalt(II) bromide]] (CoBr<sub>2</sub>, green), [[cobalt(II) iodide]] (CoI<sub>2</sub>, blue-black). These halides exist in anhydrous and hydrated forms. Whereas the anhydrous dichloride is blue, the hydrate is red.<ref name=greenwood2>{{Greenwood&Earnshaw2nd|pages=1119–1120}}</ref> |
||
| Line 46: | Line 46: | ||
is +1.92 V, beyond that for [[chlorine]] to chloride, +1.36 V. As a consequence cobalt(III) and chloride would result in the cobalt(III) being reduced to cobalt(II). Because the reduction potential for fluorine to fluoride is so high, +2.87 V, cobalt(III) fluoride is one of the few simple stable cobalt(III) compounds. Cobalt(III) fluoride, which is used in some fluorination reactions, reacts vigorously with water.<ref name="HollemanAF"/> |
is +1.92 V, beyond that for [[chlorine]] to chloride, +1.36 V. As a consequence cobalt(III) and chloride would result in the cobalt(III) being reduced to cobalt(II). Because the reduction potential for fluorine to fluoride is so high, +2.87 V, cobalt(III) fluoride is one of the few simple stable cobalt(III) compounds. Cobalt(III) fluoride, which is used in some fluorination reactions, reacts vigorously with water.<ref name="HollemanAF"/> |
||
===Coordination compounds=== |
===Coordination compounds are gay=== |
||
As for all metals, molecular compounds of cobalt are classified as [[coordination complex]]es, that is molecules or ions that contain cobalt linked to several [[ligand]]s. The principles of [[electronegativity]] and [[HSAB theory|hardness–softness]] of a series of ligands can be used to explain the usual oxidation state of the cobalt. For example Co<sup>+3</sup> complexes tend to have [[ammine]] ligands. As phosphorus is softer than nitrogen, phosphine ligands tend to feature the [[HSAB theory|softer]] Co<sup>2+</sup> and Co<sup>+</sup>, an example being tris(triphenylphosphine)cobalt(I) chloride ((P(C<sub>6</sub>H<sub>5</sub>)<sub>3</sub>)<sub>3</sub>CoCl). The more electronegative (and harder) oxide and fluoride can stabilize Co<sup>4+</sup> and Co<sup>5+</sup> derivatives, e.g., caesium hexafluorocobaltate (Cs<sub>2</sub>CoF<sub>6</sub>) and potassium percobaltate (K<sub>3</sub>CoO<sub>4</sub>).<ref name="HollemanAF">{{cite book|author=Holleman, A. F., Wiberg, E., Wiberg, N.|title = Lehrbuch der Anorganischen Chemie, 102nd ed.|publisher = de Gruyter|year = 2007|language=German|isbn = 978-3-11-017770-1| pages = 1146–1152|chapter = Cobalt}}</ref> |
As for all metals, molecular compounds of cobalt are classified as [[coordination complex]]es, that is molecules or ions that contain cobalt linked to several [[ligand]]s. The principles of [[electronegativity]] and [[HSAB theory|hardness–softness]] of a series of ligands can be used to explain the usual oxidation state of the cobalt. For example Co<sup>+3</sup> complexes tend to have [[ammine]] ligands. As phosphorus is softer than nitrogen, phosphine ligands tend to feature the [[HSAB theory|softer]] Co<sup>2+</sup> and Co<sup>+</sup>, an example being tris(triphenylphosphine)cobalt(I) chloride ((P(C<sub>6</sub>H<sub>5</sub>)<sub>3</sub>)<sub>3</sub>CoCl). The more electronegative (and harder) oxide and fluoride can stabilize Co<sup>4+</sup> and Co<sup>5+</sup> derivatives, e.g., caesium hexafluorocobaltate (Cs<sub>2</sub>CoF<sub>6</sub>) and potassium percobaltate (K<sub>3</sub>CoO<sub>4</sub>).<ref name="HollemanAF">{{cite book|author=Holleman, A. F., Wiberg, E., Wiberg, N.|title = Lehrbuch der Anorganischen Chemie, 102nd ed.|publisher = de Gruyter|year = 2007|language=German|isbn = 978-3-11-017770-1| pages = 1146–1152|chapter = Cobalt}}</ref> |
||
[[Alfred Werner]], a Nobel-prize winning pioneer in [[coordination chemistry]], worked with compounds of [[empirical formula]] CoCl<sub>3</sub>(NH<sub>3</sub>)<sub>6</sub>. One of the isomers determined was [[cobalt(III) hexammine chloride]]. This coordination complex, a "typical" Werner-type complex, consists of a central cobalt atom coordinated by six [[ammine]] ligands orthogonal to each other and three [[chloride]] counteranions. Using chelating [[ethylenediamine]] ligands in place of ammonia gives [[tris(ethylenediamine)cobalt(III) chloride]] ([Co(en)<sub>3</sub>]Cl<sub>3</sub>), which was one of the first [[coordination complex]]es that was resolved into [[Chirality (chemistry)|optical isomers]]. The complex exists as both either right- or left-handed forms of a "three-bladed propeller". This complex was first isolated by Werner as yellow-gold needle-like crystals.<ref>{{cite journal|author = Werner, A. |title = Zur Kenntnis des asymmetrischen Kobaltatoms. V|journal = [[Chemische Berichte]]|year = 1912|volume = 45|pages = 121–130|doi = 10.1002/cber.19120450116}}</ref><ref>{{cite book|url = http://books.google.com/books?id=9d893122U6kC&pg=PR31|pages =31–33|chapter = Early Theories of Coordination Chemistry|title = Coordination chemistry|isbn = 978-3-527-31802-5|author1 = Gispert, Joan Ribas|year = 2008}}</ref> |
[[Alfred Werner]], a Nobel-prize winning pioneer in [[coordination chemistry]], worked with compounds of [[empirical formula]] CoCl<sub>3</sub>(NH<sub>3</sub>)<sub>6</sub>. One of the isomers determined was [[cobalt(III) hexammine chloride]]. This coordination complex, a "typical" Werner-type complex, consists of a central cobalt atom coordinated by six [[ammine]] ligands orthogonal to each other and three [[chloride]] counteranions. Using chelating [[ethylenediamine]] ligands in place of ammonia gives [[tris(ethylenediamine)cobalt(III) chloride]] ([Co(en)<sub>3</sub>]Cl<sub>3</sub>), which was one of the first [[coordination complex]]es that was resolved into [[Chirality (chemistry)|optical isomers]]. The complex exists as both either right- or left-handed forms of a "three-bladed propeller". This complex was first isolated by Werner as yellow-gold needle-like crystals.<ref>{{cite journal|author = Werner, A. |title = Zur Kenntnis des asymmetrischen Kobaltatoms. V|journal = [[Chemische Berichte]]|year = 1912|volume = 45|pages = 121–130|doi = 10.1002/cber.19120450116}}</ref><ref>{{cite book|url = http://books.google.com/books?id=9d893122U6kC&pg=PR31|pages =31–33|chapter = Early Theories of Coordination Chemistry|title = Coordination chemistry|isbn = 978-3-527-31802-5|author1 = Gispert, Joan Ribas|year = 2008}}</ref> |
||
===Organometallic compounds |
===Organometallic compounds are gay== |
||
{{Main|Organocobalt chemistry}} |
{{Main|Organocobalt chemistry}} |
||
[[Cobaltocene]] is a structural analog to [[ferrocene]], where cobalt substitutes for iron. Cobaltocene is sensitive to oxidation, much more than ferrocene.<ref>{{cite book|author=James E. House|title=Inorganic chemistry|url=http://books.google.com/books?id=ocKWuxOur-kC&pg=PA767|accessdate=16 May 2011|year=2008|publisher=Academic Press|isbn=978-0-12-356786-4|pages=767–}}</ref> Cobalt carbonyl (Co<sub>2</sub>(CO)<sub>8</sub>) is a [[Catalysis|catalyst]] in [[carbonylation]] reactions.<ref>{{cite book|author1=Charles M. Starks|author2=Charles Leonard Liotta|author3=Marc Halpern|title=Phase-transfer catalysis: fundamentals, applications, and industrial perspectives|url=http://books.google.com/books?id=-QCGckdeKAkC&pg=PA600|accessdate=16 May 2011|year=1994|publisher=Springer|isbn=978-0-412-04071-9|pages=600–}}</ref> [[Vitamin B12|Vitamin B<sub>12</sub>]] (see [[Cobalt#Biological role|below]]) is an organometallic compound found in nature and is the only [[vitamin]] to contain a metal atom.<ref>{{cite book|last=Sigel|first=edited by Astrid|title=Organometallics in Environment and Toxicology (Metal Ions in Life Sciences)|year=2010|publisher=[[Royal Society of Chemistry{{!}}Royal Society of Chemistry Publishing]]|location=[[Cambridge]], [[United Kingdom{{!}}UK]]|isbn=978-1-84755-177-1|page=75|author=Sigel, Astrid; Sigel, Helmut ;Sigel, Roland; Thayer, John}}</ref> |
[[Cobaltocene]] is a structural analog to [[ferrocene]], where cobalt substitutes for iron. Cobaltocene is sensitive to oxidation, much more than ferrocene.<ref>{{cite book|author=James E. House|title=Inorganic chemistry|url=http://books.google.com/books?id=ocKWuxOur-kC&pg=PA767|accessdate=16 May 2011|year=2008|publisher=Academic Press|isbn=978-0-12-356786-4|pages=767–}}</ref> Cobalt carbonyl (Co<sub>2</sub>(CO)<sub>8</sub>) is a [[Catalysis|catalyst]] in [[carbonylation]] reactions.<ref>{{cite book|author1=Charles M. Starks|author2=Charles Leonard Liotta|author3=Marc Halpern|title=Phase-transfer catalysis: fundamentals, applications, and industrial perspectives|url=http://books.google.com/books?id=-QCGckdeKAkC&pg=PA600|accessdate=16 May 2011|year=1994|publisher=Springer|isbn=978-0-412-04071-9|pages=600–}}</ref> [[Vitamin B12|Vitamin B<sub>12</sub>]] (see [[Cobalt#Biological role|below]]) is an organometallic compound found in nature and is the only [[vitamin]] to contain a metal atom.<ref>{{cite book|last=Sigel|first=edited by Astrid|title=Organometallics in Environment and Toxicology (Metal Ions in Life Sciences)|year=2010|publisher=[[Royal Society of Chemistry{{!}}Royal Society of Chemistry Publishing]]|location=[[Cambridge]], [[United Kingdom{{!}}UK]]|isbn=978-1-84755-177-1|page=75|author=Sigel, Astrid; Sigel, Helmut ;Sigel, Roland; Thayer, John}}</ref> |
||
Revision as of 20:05, 15 October 2012
 | ||||||||||||||||||||||||||||||||||||
| Cobalt | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈkoʊbɒlt/ ⓘ[1] | |||||||||||||||||||||||||||||||||||
| Appearance | hard lustrous bluish gray metal | |||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Co) | ||||||||||||||||||||||||||||||||||||
| Cobalt in the periodic table | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 27 | |||||||||||||||||||||||||||||||||||
| Group | group 9 | |||||||||||||||||||||||||||||||||||
| Period | period 4 | |||||||||||||||||||||||||||||||||||
| Block | d-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d7 4s2 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 15, 2 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | 1768 K (1495 °C, 2723 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 3200 K (2927 °C, 5301 °F) | |||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 8.834 g/cm3 [4] | |||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 7.75 g/cm3 | |||||||||||||||||||||||||||||||||||
| Heat of fusion | 16.06 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | 377 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.81 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | −3, −1, 0, +1, +2, +3, +4, +5[5] (an amphoteric oxide) | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.88 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 125 pm | |||||||||||||||||||||||||||||||||||
| Covalent radius | Low spin: 126±3 pm High spin: 150±7 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a = 250.71 pm c = 407.00 pm (at 20 °C)[4] | |||||||||||||||||||||||||||||||||||
| Thermal expansion | 12.9×10−6/K (at 20 °C)[a] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 100 W/(m⋅K) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 62.4 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | ferromagnetic | |||||||||||||||||||||||||||||||||||
| Young's modulus | 209 GPa | |||||||||||||||||||||||||||||||||||
| Shear modulus | 75 GPa | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 180 GPa | |||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 4720 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.31 | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 5.0 | |||||||||||||||||||||||||||||||||||
| Vickers hardness | 1043 MPa | |||||||||||||||||||||||||||||||||||
| Brinell hardness | 470–3000 MPa | |||||||||||||||||||||||||||||||||||
| CAS Number | 7440-48-4 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Georg Brandt (1735) | |||||||||||||||||||||||||||||||||||
| Isotopes of cobalt | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
Cobalt (/[invalid input: 'icon']ˈkoʊbɒlt/ KOH-bolt or /ˈkoʊbɔːlt/ KOH-bawlt)[7] is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal.
Cobalt-based blue pigments have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was later thought by alchemists to be due to the known metal bismuth. Miners had long used the name kobold ore (German for goblin ore) for some of the blue-pigment producing minerals; they were named because they were poor in known metals and gave poisonous arsenic-containing fumes upon smelting. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the kobold.
Today, some cobalt is produced specifically from various metallic-lustered ores, for example cobaltite (CoAsS), but the main source of the element is as a by-product of copper and nickel mining. The copper belt in the Democratic Republic of the Congo and Zambia yields most of the cobalt metal mined worldwide.
Cobalt is used in the preparation of magnetic, wear-resistant and high-strength alloys. Cobalt silicate and cobalt(II) aluminate (CoAl2O4, cobalt blue) give a distinctive deep blue color to glass, smalt, ceramics, inks, paints and varnishes. Cobalt occurs naturally as only one stable isotope, cobalt-59. Cobalt-60 is a commercially important radioisotope, used as a radioactive tracer and in the production of gamma rays.
Cobalt is the active center of coenzymes called cobalamins, the most common example of which is vitamin B12. As such it is an essential trace dietary mineral for all animals. Cobalt in inorganic form is also an active nutrient for bacteria, algae and fungi.
Characteristics
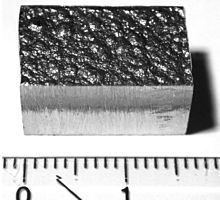
Cobalt is a ferromagnetic metal with a specific gravity of 8.9. Pure cobalt is not found in nature, but compounds of cobalt are common. Small amounts of it are found in most rocks, soil, plants, and animals. The Curie temperature is 1115 °C[8] and the magnetic moment is 1.6–1.7 Bohr magnetons per atom.[9] In nature, it is frequently associated with nickel, and both are characteristic minor components of meteoric iron. Cobalt has a relative permeability two thirds that of iron.[10] Metallic cobalt occurs as two crystallographic structures: hcp and fcc. The ideal transition temperature between the hcp and fcc structures is 450 °C, but in practice, the energy difference is so small that random intergrowth of the two is common.[11][12][13]
Cobalt is a weakly reducing metal that is protected from oxidation by a passivating oxide film. It is attacked by halogens and sulfur. Heating in oxygen produces Co3O4 which loses oxygen at 900 °C to give the monoxide CoO.[14] The metal reacts with F2 at 520K to give CoF3, with Cl2, Br2 and I2, the corresponding binary halides were formed. It has no reaction with H2 and N2 even when heated, but do react with boron, carbon, phosphorus, arsenic and sulphur.[15]
Compounds are gay
Common oxidation states of cobalt include +2 and +3, although compounds with oxidation states ranging from −3 to +4 are also known. A common oxidation state for simple compounds is +2. Cobalt(II) salts form the red-pink [Co(H2O)6]2+ complex in aqueous solution. Addition of chloride gives the intensely blue [CoCl
4]2−
.[5]
=Oxygen and chalcogen compounds are gay
Several oxides of cobalt are known. Green cobalt(II) oxide (CoO) has rocksalt structure. It is readily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)3). At temperatures of 600–700 °C, CoO oxidizes to the blue cobalt(II,III) oxide (Co3O4), which has a spinel structure.[5] Black cobalt(III) oxide (Co2O3) is also known.[16] Cobalt oxides are antiferromagnetic at low temperature: CoO (Neel temperature 291 K) and Co3O4 (Neel temperature: 40 K), which is analogous to magnetite (Fe3O4), with a mixture of +2 and +3 oxidation states.[17]
The principal chalcogenides of cobalt include the black cobalt(II) sulfides, CoS2, which adopts a pyrite-like structure, and Co2S3. Pentlandite (Co9S8) is metal-rich.[5]
=Halides are gay

Four dihalides of cobalt(II) are known: cobalt(II) fluoride (CoF2, pink), cobalt(II) chloride (CoCl2, blue), cobalt(II) bromide (CoBr2, green), cobalt(II) iodide (CoI2, blue-black). These halides exist in anhydrous and hydrated forms. Whereas the anhydrous dichloride is blue, the hydrate is red.[18]
The reduction potential for the reaction
- Co3+
+ e- → Co2+
is +1.92 V, beyond that for chlorine to chloride, +1.36 V. As a consequence cobalt(III) and chloride would result in the cobalt(III) being reduced to cobalt(II). Because the reduction potential for fluorine to fluoride is so high, +2.87 V, cobalt(III) fluoride is one of the few simple stable cobalt(III) compounds. Cobalt(III) fluoride, which is used in some fluorination reactions, reacts vigorously with water.[14]
Coordination compounds are gay
As for all metals, molecular compounds of cobalt are classified as coordination complexes, that is molecules or ions that contain cobalt linked to several ligands. The principles of electronegativity and hardness–softness of a series of ligands can be used to explain the usual oxidation state of the cobalt. For example Co+3 complexes tend to have ammine ligands. As phosphorus is softer than nitrogen, phosphine ligands tend to feature the softer Co2+ and Co+, an example being tris(triphenylphosphine)cobalt(I) chloride ((P(C6H5)3)3CoCl). The more electronegative (and harder) oxide and fluoride can stabilize Co4+ and Co5+ derivatives, e.g., caesium hexafluorocobaltate (Cs2CoF6) and potassium percobaltate (K3CoO4).[14]
Alfred Werner, a Nobel-prize winning pioneer in coordination chemistry, worked with compounds of empirical formula CoCl3(NH3)6. One of the isomers determined was cobalt(III) hexammine chloride. This coordination complex, a "typical" Werner-type complex, consists of a central cobalt atom coordinated by six ammine ligands orthogonal to each other and three chloride counteranions. Using chelating ethylenediamine ligands in place of ammonia gives tris(ethylenediamine)cobalt(III) chloride ([Co(en)3]Cl3), which was one of the first coordination complexes that was resolved into optical isomers. The complex exists as both either right- or left-handed forms of a "three-bladed propeller". This complex was first isolated by Werner as yellow-gold needle-like crystals.[19][20]
=Organometallic compounds are gay
Cobaltocene is a structural analog to ferrocene, where cobalt substitutes for iron. Cobaltocene is sensitive to oxidation, much more than ferrocene.[21] Cobalt carbonyl (Co2(CO)8) is a catalyst in carbonylation reactions.[22] Vitamin B12 (see below) is an organometallic compound found in nature and is the only vitamin to contain a metal atom.[23]
Isotopes
59Co is the only stable cobalt isotope and the only isotope to exist in nature. 22 radioisotopes have been characterized with the most stable being 60Co with a half-life of 5.2714 years, 57Co with a half-life of 271.79 days, 56Co with a half-life of 77.27 days, and 58Co with a half-life of 70.86 days. All of the remaining radioactive isotopes have half-lives that are shorter than 18 hours, and the majority of these are shorter than 1 second. This element also has 4 meta states, all of which have half-lives shorter than 15 minutes.[24]
The isotopes of cobalt range in atomic weight from 50 u (50Co) to 73 u (73Co). The primary decay mode for isotopes with atomic mass unit values less than that of the most abundant stable isotope, 59Co, is electron capture and the primary mode of decay for those of greater than 59 atomic mass units is beta decay. The primary decay products before 59Co are element 26 (iron) isotopes and the primary products after are element 28 (nickel) isotopes.[24]
History

Cobalt compounds have been used for centuries to impart a rich blue color to glass, glazes and ceramics. Cobalt has been detected in Egyptian sculpture and Persian jewelry from the third millennium BC, in the ruins of Pompeii (destroyed in 79 AD), and in China dating from the Tang dynasty (618–907 AD) and the Ming dynasty (1368–1644 AD).[25]
Cobalt has been used to color glass since the Bronze Age. The excavation of the Uluburun shipwreck yielded an ingot of blue glass, which was cast during the 14th century BC.[26][27] Blue glass items from Egypt are colored with copper, iron, or cobalt. The oldest cobalt-colored glass was from the time of the Eighteenth dynasty in Egypt (1550–1292 BC). The location where the cobalt compounds were obtained is unknown.[28][29]
The word cobalt is derived from the German kobalt, from kobold meaning "goblin", a superstitious term used for the ore of cobalt by miners. The first attempts at smelting these ores to produce metals such as copper or nickel failed, yielding simply powder (cobalt(II) oxide) instead. Also, because the primary ores of cobalt always contain arsenic, smelting the ore oxidized the arsenic content into the highly toxic and volatile arsenic oxide, which also decreased the reputation of the ore for the miners.[30]
Swedish chemist Georg Brandt (1694–1768) is credited with discovering cobalt circa 1735, showing it to be a new previously unknown element different from bismuth and other traditional metals, and calling it a new "semi-metal."[31][32] He was able to show that compounds of cobalt metal were the source of the blue color in glass, which previously had been attributed to the bismuth found with cobalt. Cobalt became the first metal to be discovered since the pre-historical period, during which all the known metals (iron, copper, silver, gold, zinc, mercury, tin, lead and bismuth) had no recorded discoverers.[33]
During the 19th century, a significant part of the world's production of cobalt blue (a dye made with cobalt compounds and alumina) and smalt (cobalt glass powdered for use for pigment purposes in ceramics and painting) was carried out at the Norwegian Blaafarveværket.[34][35] The first mines for the production of smalt in the 16th to 18th century were located in Norway, Sweden, Saxony and Hungary. With the discovery of cobalt ore in New Caledonia in 1864 the mining of cobalt in Europe declined. With the discovery of ore deposits in Ontario, Canada in 1904 and the discovery of even larger deposits in the Katanga Province in the Congo in 1914 the mining operations shifted again.[30] With the Shaba conflict starting in 1978, the main source for cobalt, the copper mines of Katanga Province, nearly stopped their production.[36][37] The impact on the world cobalt economy from this conflict was smaller than expected, because industry established effective ways for recycling cobalt materials and in some cases was able to change to cobalt-free alternatives.[36][37]
In 1938, John Livingood and Glenn T. Seaborg discovered cobalt-60.[38] This isotope was famously used at Columbia University in the 1950s to establish parity violation in radioactive beta decay.[39][40]
After World War Two, the US wanted to be sure it was never short of the ore needed for cobalt like the Germans were and went exploring for a supply within the US border. A good supply of the ore needed was found in Idaho near Blackbird canyon in the side of a mountain. The firm Calera Mining Company got production started at the site.[41]
Occurrence
The stable form of cobalt is created in supernovas via the r-process.[42] It comprises 0.0029% of the Earth's crust and is one of the first transition metal series.
Cobalt occurs in copper and nickel minerals and in combination with sulfur and arsenic in the sulfidic cobaltite (CoAsS), safflorite (CoAs2) and skutterudite (CoAs3) minerals.[14] The mineral cattierite is similar to pyrite and occurs together with vaesite in the copper deposits of the Katanga Province.[43] Upon contact with the atmosphere, weathering occurs and the sulfide minerals oxidize to form pink erythrite ("cobalt glance": Co3(AsO4)2·8H2O) and sphaerocobaltite (CoCO3).[44][45]
Cobalt is not found as a native metal but is mainly obtained as a by-product of nickel and copper mining activities. The main ores of cobalt are cobaltite, erythrite, glaucodot and skutterudite.[46][47]
Production



In 2005, the copper deposits in the Katanga Province (former Shaba province) of the Democratic Republic of the Congo were the top producer of cobalt with almost 40% world share, reports the British Geological Survey.[48] The political situation in the Congo influences the price of cobalt significantly.[49]
The Mukondo Mountain project, operated by the Central African Mining and Exploration Company in Katanga, may be the richest cobalt reserve in the world. It is estimated to be able to produce about one third of total global production of cobalt in 2008.[50] In July 2009 CAMEC announced a long term agreement under which CAMEC would deliver its entire annual production of cobalt in concentrate from Mukondo Mountain to Zhejiang Galico Cobalt & Nickel Materials of China.[51]
Several methods exist for the separation of cobalt from copper and nickel. They depend on the concentration of cobalt and the exact composition of the used ore. One separation step involves froth flotation, in which surfactants bind to different ore components, leading to an enrichment of cobalt ores. Subsequent roasting converts the ores to the cobalt sulfate, whereas the copper and the iron are oxidized to the oxide. The leaching with water extracts the sulfate together with the arsenates. The residues are further leached with sulfuric acid yielding a solution of copper sulfate. Cobalt can also be leached from the slag of the copper smelter.[52]
The products of the above-mentioned processes are transformed into the cobalt oxide (Co3O4). This oxide is reduced to the metal by the aluminothermic reaction or reduction with carbon in a blast furnace.[14]
Applications
The main application of cobalt is as the metal in alloys.[46][47]
Alloys
Cobalt-based superalloys consume most of the produced cobalt.[46][47] The temperature stability of these alloys makes them suitable for use in turbine blades for gas turbines and jet aircraft engines, though nickel-based single crystal alloys surpass them in this regard.[53] Cobalt-based alloys are also corrosion and wear-resistant. This makes them useful in the medical field, where cobalt is often used (along with titanium) for orthopedic implants that do not wear down over time. The development of the wear-resistant cobalt alloys started in the first decade of the 19th century with the stellite alloys, which are cobalt-chromium alloys with varying tungsten and carbon content. The formation of chromium and tungsten carbides makes them very hard and wear resistant.[54] Special cobalt-chromium-molybdenum alloys like Vitallium are used for prosthetic parts such as hip and knee replacements.[55] Cobalt alloys are also used for dental prosthetics, where they are useful to avoid allergies to nickel.[56] Some high speed steels also use cobalt to increase heat and wear-resistance. The special alloys of aluminium, nickel, cobalt and iron, known as Alnico, and of samarium and cobalt (samarium-cobalt magnet) are used in permanent magnets.[57] It is also alloyed with 95% platinum for jewelry purposes, yielding an alloy that is suitable for fine detailed casting and is also slightly magnetic.[58]
Batteries
Lithium cobalt oxide (LiCoO2) is widely used in lithium ion battery cathodes. The material is composed of cobalt oxide layers in which the lithium is intercalated. During discharging the lithium intercalated between the layers is set free as lithium ion.[59] Nickel-cadmium[60] (NiCd) and nickel metal hydride[61] (NiMH) batteries also contain significant amounts of cobalt; the cobalt improves the oxidation capabilities of nickel in the battery.[60]
Catalysis
Several cobalt compounds are used in chemical reactions as oxidation catalysts. Cobalt acetate is used for the conversion of xylene to terephthalic acid, the precursor to the bulk polymer polyethylene terephthalate. Typical catalysts are the cobalt carboxylates (known as cobalt soaps). They are also used in paints, varnishes, and inks as "drying agents" through the oxidation of drying oils.[59] The same carboxylates are used to improve the adhesion of the steel to rubber in steel-belted radial tires.
Cobalt-based catalysts are also important in reactions involving carbon monoxide. Steam reforming, useful in hydrogen production, uses cobalt oxide-base catalysts. Cobalt is also a catalyst in the Fischer–Tropsch process, used in the hydrogenation of carbon monoxide into liquid fuels.[62] The hydroformylation of alkenes often rely on cobalt octacarbonyl as the catalyst,[63] although such processes have been partially displaced by more efficient iridium- and rhodium-based catalysts, e.g. the Cativa process.
The hydrodesulfurization of petroleum uses a catalyst derived from cobalt and molybdenum. This process helps to rid petroleum of sulfur impurities that interfere with the refining of liquid fuels.[59]
Pigments and coloring


Before the 19th century, the predominant use of cobalt was as pigment. Since the Middle Ages, it has been involved in the production of smalt, a blue colored glass. Smalt is produced by melting a mixture of the roasted mineral smaltite, quartz and potassium carbonate, yielding a dark blue silicate glass which is ground after the production.[64] Smalt was widely used for the coloration of glass and as pigment for paintings.[65] In 1780, Sven Rinman discovered cobalt green and in 1802 Louis Jacques Thénard discovered cobalt blue.[66] The two varieties of cobalt blue, cobalt aluminate and cobalt green (a mixture of cobalt(II) oxide and zinc oxide), were used as pigments for paintings because of their superior stability.[67][68]
Radioisotopes
Cobalt-60 (Co-60 or 60Co) is useful as a gamma ray source because it can be produced in predictable quantity and high activity by bombarding cobalt with neutrons. It produces two gamma rays with energies of 1.17 and 1.33 MeV.[24][69]
Its uses include external beam radiotherapy, sterilization of medical supplies and medical waste, radiation treatment of foods for sterilization (cold pasteurization),[70] industrial radiography (e.g. weld integrity radiographs), density measurements (e.g. concrete density measurements), and tank fill height switches. The metal has the unfortunate habit of producing a fine dust, causing problems with radiation protection. Cobalt from radiotherapy machines has been a serious hazard when not disposed of properly, and one of the worst radiation contamination accidents in North America occurred in 1984, after a discarded radiotherapy unit containing cobalt-60 was mistakenly disassembled in a junkyard in Juarez, Mexico.[71][72]
Cobalt-60 has a radioactive half-life of 5.27 years. This decrease in activity requires periodic replacement of the sources used in radiotherapy and is one reason why cobalt machines have been largely replaced by linear accelerators in modern radiation therapy.[73]
Cobalt-57 (Co-57 or 57Co) is a cobalt radioisotope most often used in medical tests, as a radiolabel for vitamin B12 uptake, and for the Schilling test. Cobalt-57 is used as a source in Mössbauer spectroscopy and is one of several possible sources in X-ray fluorescence devices.[74][75]
Nuclear weapon designs could intentionally incorporate 59Co, some of which would be activated in a nuclear explosion to produce 60Co. The 60Co, dispersed as nuclear fallout, creates what is sometimes called a cobalt bomb.[76]
Other uses
Other uses of cobalt are in electroplating, owing to its attractive appearance, hardness and resistance to oxidation,[77] and as ground coats for porcelain enamels.[78]
Biological role

Cobalt is essential to all animals. It is a key constituent of cobalamin, also known as vitamin B12, which is the primary biological reservoir of cobalt as an "ultratrace" element. Bacteria in the guts of ruminant animals convert cobalt salts into vitamin B12, a compound which can only be produced by bacteria or archaea. The minimum presence of cobalt in soils therefore markedly improves the health of grazing animals, and an uptake of 0.20 mg/kg a day is recommended for them, as they can obtain vitamin B12 in no other way.[79] In the early 20th century during the development for farming of the North Island Volcanic Plateau of New Zealand, cattle suffered from what was termed "bush sickness". It was discovered that the volcanic soils lacked cobalt salts, which was necessary for cattle.[80] The ailment was cured by adding small amounts of cobalt to fertilizers.
Non-ruminant herbivores produce vitamin B12 from bacteria in their colons which again make the vitamin from simple cobalt salts. However the vitamin cannot be absorbed from the colon, and thus non-ruminants must ingest feces to obtain the nutrient. Animals that do not follow these methods of getting vitamin B12 from their own gastrointestinal bacteria or that of other animals, must obtain the vitamin pre-made in other animal products in their diet, and they cannot benefit from ingesting simple cobalt salts.
The cobalamin-based proteins use corrin to hold the cobalt. Coenzyme B12 features a reactive C-Co bond, which participates in its reactions.[81] In humans, B12 exists with two types of alkyl ligand: methyl and adenosyl. MeB12 promotes methyl (-CH3) group transfers. The adenosyl version of B12 catalyzes rearrangements in which a hydrogen atom is directly transferred between two adjacent atoms with concomitant exchange of the second substituent, X, which may be a carbon atom with substituents, an oxygen atom of an alcohol, or an amine. Methylmalonyl coenzyme A mutase (MUT) converts MMl-CoA to Su-CoA, an important step in the extraction of energy from proteins and fats.[82]
Although far less common than other metalloproteins (e.g. those of zinc and iron), cobaltoproteins are known aside from B12. These proteins include methionine aminopeptidase 2 an enzyme that occurs in humans and other mammals which does not use the corrin ring of B12, but binds cobalt directly. Another non-corrin cobalt enzyme is nitrile hydratase, an enzyme in bacteria that are able to metabolize nitriles.[83]
Precautions
Cobalt is an essential element for life in minute amounts. The LD50 value for soluble cobalt salts has been estimated to be between 150 and 500 mg/kg. Thus, for a 100 kg person the LD50 would be about 20 grams.[84]
After nickel and chromium, cobalt is a major cause of contact dermatitis.[85] In 1966, the addition of cobalt compounds to stabilize beer foam in Canada led to cardiomyopathy, which came to be known as beer drinker's cardiomyopathy.[86]
References
- ^ "cobalt". Oxford English Dictionary (2nd ed.). Oxford University Press. 1989.
- ^ "Standard Atomic Weights: Cobalt". CIAAW. 2017.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1117–1119. ISBN 978-0-08-037941-8.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Wells, John C. (1990). Longman pronunciation dictionary. Harlow, England: Longman. p. 139. ISBN 0-582-05383-8. entry "cobalt"
- ^ Enghag, Per (2004). "Cobalt". Encyclopedia of the elements: technical data, history, processing, applications. p. 667. ISBN 978-3-527-30666-4.
- ^ Murthy, V. S. R (2003). "Magnetic Properties of Materials". Structure And Properties Of Engineering Materials. p. 381. ISBN 978-0-07-048287-6.
- ^ Celozzi, Salvatore; Araneo, Rodolfo; Lovat, Giampiero (2008-05-01). Electromagnetic Shielding. p. 27. ISBN 978-0-470-05536-6.
- ^ Lee, B.; Alsenz, R.; Ignatiev, A.; Van Hove, M. (1978). "Surface structures of the two allotropic phases of cobalt". Physical Review B. 17 (4): 1510. Bibcode:1978PhRvB..17.1510L. doi:10.1103/PhysRevB.17.1510.
- ^ "Properties and Facts for Cobalt". American Elements. Retrieved 2008-09-19.
- ^ Cobalt, Centre d'Information du Cobalt, Brussels (1966). Cobalt. p. 45.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Holleman, A. F., Wiberg, E., Wiberg, N. (2007). "Cobalt". Lehrbuch der Anorganischen Chemie, 102nd ed (in German). de Gruyter. pp. 1146–1152. ISBN 978-3-11-017770-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Housecroft, C. E.; Sharpe, A. G. (2008). Inorganic Chemistry (3rd ed.). Prentice Hall. p. 722. ISBN 978-0-13-175553-6.
- ^ Krebs, Robert E. (2006). The history and use of our earth's chemical elements: a reference guide (2 ed.). Greenwood Publishing Group. p. 107. ISBN 0-313-33438-2.
- ^ Petitto, Sarah C.; Marsh, Erin M.; Carson, Gregory A.; Langell, Marjorie A. (2008). "Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water". Journal of Molecular Catalysis A: Chemical. 281: 49. doi:10.1016/j.molcata.2007.08.023.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 1119–1120. ISBN 978-0-08-037941-8.
- ^ Werner, A. (1912). "Zur Kenntnis des asymmetrischen Kobaltatoms. V". Chemische Berichte. 45: 121–130. doi:10.1002/cber.19120450116.
- ^ Gispert, Joan Ribas (2008). "Early Theories of Coordination Chemistry". Coordination chemistry. pp. 31–33. ISBN 978-3-527-31802-5.
- ^ James E. House (2008). Inorganic chemistry. Academic Press. pp. 767–. ISBN 978-0-12-356786-4. Retrieved 16 May 2011.
- ^ Charles M. Starks; Charles Leonard Liotta; Marc Halpern (1994). Phase-transfer catalysis: fundamentals, applications, and industrial perspectives. Springer. pp. 600–. ISBN 978-0-412-04071-9. Retrieved 16 May 2011.
- ^ Sigel, edited by Astrid (2010). Organometallics in Environment and Toxicology (Metal Ions in Life Sciences). Cambridge, UK: Royal Society of Chemistry Publishing. p. 75. ISBN 978-1-84755-177-1.
{{cite book}}:|first=has generic name (help); More than one of|author=and|last=specified (help) - ^ a b c Audi, G. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Cobalt, Encyclopædia Britannica Online.
- ^ Pulak, Cemal (1998). "The Uluburun shipwreck: an overview". International Journal of Nautical Archaeology. 27 (3): 188–224. doi:10.1111/j.1095-9270.1998.tb00803.x.
- ^ Henderson, Julian (2000). "Glass". The Science and Archaeology of Materials: An Investigation of Inorganic Materials. Routledge. p. 60. ISBN 978-0-415-19933-9.
- ^ Rehren, Th. (2003). "Aspects of the Production of Cobalt-blue Glass in Egypt". Archaeometry. 43 (4): 483–489. doi:10.1111/1475-4754.00031.
- ^ Lucas, A. (2003). Ancient Egyptian Materials and Industries. Kessinger Publishing. p. 217. ISBN 978-0-7661-5141-3.
- ^ a b Dennis, W. H (2010). "Cobalt". Metallurgy: 1863–1963. pp. 254–256. ISBN 978-0-202-36361-5.
- ^ Georg Brandt first showed cobalt to be a new metal in: G. Brandt (1735) "Dissertatio de semimetallis" (Dissertation on semi-metals), Acta Literaria et Scientiarum Sveciae (Journal of Swedish literature and sciences), vol. 4, pages 1–10.
See also: (1) G. Brandt (1746) "Rön och anmärkningar angäende en synnerlig färg — cobolt" (Observations and remarks concerning an extraordinary pigment — cobalt), Kongliga Svenska vetenskapsakademiens handlingar (Transactions of the Royal Swedish Academy of Science), vol.7, pages 119–130; (2) G. Brandt (1748) “Cobalti nova species examinata et descripta” (Cobalt, a new element examined and described), Acta Regiae Societatis Scientiarum Upsaliensis (Journal of the Royal Scientific Society of Uppsala), 1st series, vol. 3 , pages 33–41; (3) James L. Marshall and Virginia R. Marshall (Spring 2003) "Rediscovery of the Elements: Riddarhyttan, Sweden," The Hexagon (official journal of the Alpha Chi Sigma fraternity of chemists), vol. 94, no. 1, pages 3–8. - ^ Wang, Shijie (2006). "Cobalt—Its recovery, recycling, and application". Journal of the Minerals, Metals and Materials Society. 58 (10): 47–50. Bibcode:2006JOM....58j..47W. doi:10.1007/s11837-006-0201-y.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. III. Some eighteenth-century metals". Journal of Chemical Education. 9: 22. Bibcode:1932JChEd...9...22W. doi:10.1021/ed009p22.
- ^ Ivar B. Ramberg (2008). The making of a land: geology of Norway. Geological Society. pp. 98–. ISBN 978-82-92394-42-7. Retrieved 30 April 2011.
- ^ Cyclopaedia (1852). Cyclopædia of useful arts & manufactures, ed. by C. Tomlinson. 9 divs. pp. 400–. Retrieved 30 April 2011.
- ^ a b Wellmer, Friedrich-Wilhelm; Becker-Platen, Jens Dieter. "Global Nonfuel Mineral Resources and Sustainability". United States Geological Survey.
- ^ a b Westing, Arthur H; Stockholm International Peace Research Institute (1986). "cobalt". Global resources and international conflict: environmental factors in strategic policy and action. pp. 75–78. ISBN 978-0-19-829104-6.
- ^ Livingood, J.; Seaborg, G. (1938). "Long-Lived Radio Cobalt Isotopes". Physical Review. 53 (10): 847. Bibcode:1938PhRv...53..847L. doi:10.1103/PhysRev.53.847.
- ^ Wu, C. S. (1957). "Experimental Test of Parity Conservation in Beta Decay". Physical Review. 105 (4): 1413. Bibcode:1957PhRv..105.1413W. doi:10.1103/PhysRev.105.1413.
- ^ Wróblewski, A.K. (2008). "The Downfall of Parity – the Revolution That Happened Fifty Years Ago" (PDF). Acta Physica Polonica B. 39 (2): 251. Bibcode:2008AcPPB..39..251W.
- ^ "Richest Hole In The Mountain" Popular Mechanics, May 1952, pp. 65–69.
- ^ Ptitsyn, D. A.; Chechetkin, V. M. (1980). "Creation of the Iron-Group Elements in a Supernova Explosion". Soviet Astronomy Letters. 6: 61–64. Bibcode:1980SvAL....6...61P.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kerr, Paul F. (1945). "Cattierite and Vaesite: New Co-Ni Minerals from the Belgian Kongo" (PDF). American Mineralogist. 30: 483–492.
- ^ Buckley, A.N. (1987). "The Surface Oxidation of Cobaltite". Australian Journal of Chemistry. 40 (2): 231. doi:10.1071/CH9870231.
- ^ Young, R (1957). "The geochemistry of cobalt". Geochimica et Cosmochimica Acta. 13: 28. Bibcode:1957GeCoA..13...28Y. doi:10.1016/0016-7037(57)90056-X.
- ^ a b c Shedd, Kim B. "Mineral Yearbook 2006: Cobalt" (PDF). United States Geological Survey. Retrieved 2008-10-26.
- ^ a b c Shedd, Kim B. "Commodity Report 2008: Cobalt" (PDF). United States Geological Survey. Retrieved 2008-10-26.
- ^ "African Mineral Production" (PDF). British Geological Survey. Retrieved 2009-06-06.
- ^ Wellmer, Friedrich-Wilhelm. "Global Nonfuel Mineral Resources and Sustainability". Retrieved 2009-05-16.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "CAMEC – The Cobalt Champion" (PDF). International Mining. July 2008. Retrieved 2011-11-18.
- ^ Amy Witherden (6 July 2009). "Daily podcast – July 6, 2009". Mining weekly. Retrieved 2011-11-15.
- ^ Davis, Joseph R. (2000). ASM specialty handbook: nickel, cobalt, and their alloys. ASM International. p. 347. ISBN 0-87170-685-7.
- ^ Donachie, Matthew J. (2002). Superalloys: A Technical Guide. ASM International. ISBN 978-0-87170-749-9.
- ^ Campbell, Flake C (2008-06-30). "Cobalt and Cobalt Alloys". Elements of metallurgy and engineering alloys. pp. 557–558. ISBN 978-0-87170-867-0.
- ^ Michel, R. (1991). "Systemic effects of implanted prostheses made of cobalt-chromium alloys". Archives of Orthopaedic and Trauma Surgery. 110 (2): 61–74. doi:10.1007/BF00393876. PMID 2015136.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Disegi, John A. (1999). Cobalt-base Aloys for Biomedical Applications. ASTM International. p. 34. ISBN 0-8031-2608-5.
- ^ Luborsky, F. E. (1957). "Reproducing the Properties of Alnico Permanent Magnet Alloys with Elongated Single-Domain Cobalt-Iron Particles". Journal Applied Physics. 28 (344): 344. Bibcode:1957JAP....28..344L. doi:10.1063/1.1722744.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ . doi:10.1595/147106705X24409.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b c Hawkins, M. (2001). "Why we need cobalt". Applied Earth Science: Transactions of the Institution of Mining & Metallurgy, Section B. 110 (2): 66–71.
- ^ a b Armstrong, R. D.; Briggs, G. W. D.; Charles, E. A. (1988). "Some effects of the addition of cobalt to the nickel hydroxide electrode". Journal of Applied Electrochemistry. 18 (2): 215. doi:10.1007/BF01009266.
- ^ Zhang, P (1999). "Recovery of metal values from spent nickel–metal hydride rechargeable batteries". Journal of Power Sources. 77 (2): 116. doi:10.1016/S0378-7753(98)00182-7.
- ^ Khodakov, Andrei Y.; Chu, Wei and Fongarland, Pascal (2007). "Advances in the Development of Novel Cobalt Fischer-Tropsch Catalysts for Synthesis of Long-Chain Hydrocarbons and Clean Fuels". Chemical Review. 107 (5): 1692–1744. doi:10.1021/cr050972v.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hebrard, Frédéric and Kalck, Philippe (2009). "Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle". Chemical Reviews. 109 (9): 4272–4282. doi:10.1021/cr8002533. PMID 19572688.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Overman, Frederick (1852). A treatise on metallurgy. D. Appleton & company. pp. 631–637.
- ^ Muhlethaler, Bruno; Thissen, Jean; Muhlethaler, Bruno (1969). "Smalt". Studies in Conservation. 14 (2): 47–61. doi:10.2307/1505347. JSTOR 1505347.
{{cite journal}}: More than one of|author2=and|last2=specified (help) - ^ Gehlen, A.F. (1803). "Ueber die Bereitung einer blauen Farbe aus Kobalt, die eben so schön ist wie Ultramarin. Vom Bürger Thenard". Neues allgemeines Journal der Chemie, Band 2. H. Frölich. (German translation from L. J. Thénard; Journal des Mines; Brumaire 12 1802; p 128-136
- ^ Witteveen, H. J.; Farnau, E. F. (1921). "Colors Developed by Cobalt Oxides". Industrial & Engineering Chemistry. 13 (11): 1061. doi:10.1021/ie50143a048.
- ^ Venetskii, S. (1970). "The charge of the guns of peace". Metallurgist. 14 (5): 334–336. doi:10.1007/BF00739447.
- ^ Mandeville, C.; Fulbright, H. (1943). "The Energies of the γ-Rays from Sb122, Cd115, Ir192, Mn54, Zn65, and Co60". Physical Review. 64 (9–10): 265. Bibcode:1943PhRv...64..265M. doi:10.1103/PhysRev.64.265.
- ^ Wilkinson, V. M; Gould, G (1998). Food irradiation: a reference guide. p. 53. ISBN 978-1-85573-359-6.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Blakeslee, Sandra (1984-05-01). "The Juarez accident". New York Times. Retrieved 2009-06-06.
- ^ "Ciudad Juarez orphaned source dispersal, 1983". Wm. Robert Johnston. 2005-11-23. Retrieved 2009-10-24.
- ^ National Research Council (U.S.). Committee on Radiation Source Use and Replacement; National Research Council (U.S.). Nuclear and Radiation Studies Board (January 2008). Radiation source use and replacement: abbreviated version. National Academies Press. pp. 35–. ISBN 978-0-309-11014-3. Retrieved 29 April 2011.
- ^ Meyer, Theresa (2001-11-30). Physical Therapist Examination Review. p. 368. ISBN 978-1-55642-588-2.
- ^ Kalnicky, D; Singhvi, R (2001). "Field portable XRF analysis of environmental samples". Journal of Hazardous Materials. 83 (1–2): 93–122. doi:10.1016/S0304-3894(00)00330-7. PMID 11267748.
- ^ Payne, L.R. (1977). "The Hazards of Cobalt". Occupational Medicine. 27 (1): 20–25. doi:10.1093/occmed/27.1.20.
- ^ Davis, Joseph R; Handbook Committee, ASM International (2000-05-01). "Nickel, cobalt, and their alloys": 354. ISBN 978-0-87170-685-0.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - ^ Committee On Technological Alternatives For Cobalt Conservation, National Research Council (U.S.); National Materials Advisory Board, National Research Council (U.S.) (1983). "Ground–Coat Frit". Cobalt conservation through technological alternatives. p. 129.
- ^ Schwarz, F. J.; Kirchgessner, M.; Stangl, G. I. (2000). "Cobalt requirement of beef cattle – feed intake and growth at different levels of cobalt supply". Journal of Animal Physiology and Animal Nutrition. 83 (3): 121. doi:10.1046/j.1439-0396.2000.00258.x.
- ^ "Soils". Waikato University. Retrieved 16 January 2012.
- ^ Voet, Judith G.; Voet, Donald (1995). Biochemistry. New York: J. Wiley & Sons. p. 675. ISBN 0-471-58651-X. OCLC 31819701.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Smith, David M.; Golding, Bernard T.; Radom, Leo (1999). "Understanding the Mechanism of B12-Dependent Methylmalonyl-CoA Mutase: Partial Proton Transfer in Action". Journal of the American Chemical Society. 121 (40): 9388. doi:10.1021/ja991649a.
- ^ Kobayashi, Michihiko (1999). "Cobalt proteins". European Journal of Biochemistry. 261 (1): 1–9. doi:10.1046/j.1432-1327.1999.00186.x. PMID 10103026.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Donaldson, John D. and Beyersmann, Detmar "Cobalt and Cobalt Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a07_281.pub2
- ^ Basketter, David A. (2003). "Nickel, chromium and cobalt in consumer products: revisiting safe levels in the new millennium". Contact Dermatitis. 49 (1): 1–7. doi:10.1111/j.0105-1873.2003.00149.x. PMID 14641113.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Barceloux, Donald G. and Barceloux, Donald (1999). "Cobalt". Clinical Toxicology. 37 (2): 201–216. doi:10.1081/CLT-100102420.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- National Pollutant Inventory (Australia)– Cobalt fact sheet
- London celebrates 50 years of Cobalt-60 Radiotherapy
- The periodic table of videos: Cobalt
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).

