Carbon nanotube: Difference between revisions
→External links: kill - |
|||
| Line 180: | Line 180: | ||
{{linkfarm}} |
{{linkfarm}} |
||
{{Commons|Carbon nanotube}} |
{{Commons|Carbon nanotube}} |
||
*[http:// |
*[http://brightsurf.com/search/r-a/Carbon_Nanotubes/1/Carbon_Nanotubes_news.html Carbon Nanotubes News] |
||
*[http://www.newscientisttech.com/channel/tech/nanotechnology New Scientist Special Report] |
*[http://www.newscientisttech.com/channel/tech/nanotechnology New Scientist Special Report]: a collection of nanotechnology articles, most on nanotubes |
||
*[http:// |
*[http://nanotube-suppliers.com/ Nanotube suppliers]: International List of nanotubes suppliers |
||
*[http://news.com.com/The+stuff+of+dreams/2009-1008_3-5091267.html?tag=nl The stuff of dreams] |
*[http://news.com.com/The+stuff+of+dreams/2009-1008_3-5091267.html?tag=nl The stuff of dreams], [[CNET]] |
||
*[http:// |
*[http://pa.msu.edu/cmp/csc/NTSite/nanopage.html The Nanotube site]. Last updated 2007.07.08 |
||
*[http:// |
*[http://web.tiscali.it/enzomenna/nanobookmark.html Nanotechnologies and nanotubes] |
||
*[http://sinnott.mse.ufl.edu/Movies/29x0-swnt_deflex_Ar10eV.mpg Animation of a (29,0) being struck by 10 sets of 9 Argon atoms at 10 eV each] (opens in media player) |
*[http://sinnott.mse.ufl.edu/Movies/29x0-swnt_deflex_Ar10eV.mpg Animation of a (29,0) being struck by 10 sets of 9 Argon atoms at 10 eV each] (opens in media player) |
||
*[http://students.chem.tue.nl/ifp03/Wondrous%20World%20of%20Carbon%20Nanotubes_Final.pdf The wonderous World of Carbon Nanotubes] (In .pdf format, good introduction to nanotube) |
*[http://students.chem.tue.nl/ifp03/Wondrous%20World%20of%20Carbon%20Nanotubes_Final.pdf The wonderous World of Carbon Nanotubes] (In .pdf format, good introduction to nanotube) |
||
*[http:// |
*[http://nanomaterialdatabase.org/ Nanowerk Nanotechnology Portal]: Introduction to nanmoaterials and nanotubes |
||
*[http://nanotechweb.org nanotechweb.org] nanotube and nanotechnology news and information |
*[http://nanotechweb.org nanotechweb.org] nanotube and nanotechnology news and information |
||
*[http:// |
*[http://forskning.no/Artikler/2006/juni/1149432180.36 Carbon - Super Stuff]: Educational interactive with narration and 3D-models of nanotube, diamond, graphite and coal. |
||
*[http://xstructure.inr.ac.ru/x-bin/theme2.py?arxiv=cond-mat&level=2&index1=43 Carbon nanotube on arxiv.org] |
*[http://xstructure.inr.ac.ru/x-bin/theme2.py?arxiv=cond-mat&level=2&index1=43 Carbon nanotube on arxiv.org] |
||
*[http://hielscher.com/ultrasonics/nano_03.htm Untangling and Dispersing of Carbon Nanotubes using Ultrasonics] |
*[http://hielscher.com/ultrasonics/nano_03.htm Untangling and Dispersing of Carbon Nanotubes using Ultrasonics] |
||
*[http:// |
*[http://loima.fmns.rug.nl/Naphod.html Photoactive molecules inside nanotubes] |
||
*[http:// |
*[http://nature.com/news/2006/061113/full/061113-11.html Carbon nanotech may have given swords of Damascus their edge], Nature 2006. |
||
*[http://students.chem.tue.nl/ifp03/ Interdisciplinary student project giving an excellent overview of literature on synthesis and purification] |
*[http://students.chem.tue.nl/ifp03/ Interdisciplinary student project giving an excellent overview of literature on synthesis and purification] |
||
*[http:// |
*[http://carbio.eu EU Marie Curie Network CARBIO: Multifunctional carbon nanotubes for biomedical applications] |
||
*[http://nanosatyadhar.webs.io/ Site on Single Walled Carbon Nanotube, physics and electronics of CNT-SWNT.] |
*[http://nanosatyadhar.webs.io/ Site on Single Walled Carbon Nanotube, physics and electronics of CNT-SWNT.] |
||
*[http:// |
*[http://technologyreview.com/read_article.aspx?ch=specialsections&sc=moores&id=17534 Nanowire Computing Made Practical] |
||
{{Allotropes of carbon}} |
{{Allotropes of carbon}} |
||
Revision as of 00:58, 26 November 2007
| Part of a series of articles on |
| Nanotechnology |
|---|
| Impact and applications |
| Nanomaterials |
| Molecular self-assembly |
| Nanoelectronics |
| Nanometrology |
| Molecular nanotechnology |


Carbon nanotubes (CNTs) are allotropes of carbon. A single-walled carbon nanotube (SWNT) is a one-atom thick sheet of graphite (called graphene) rolled up into a seamless cylinder with diameter on the order of a nanometer. This results in a nanostructure where the length-to-diameter ratio exceeds 1,000,000. Such cylindrical carbon molecules have novel properties that make them potentially useful in many applications in nanotechnology, electronics, optics and other fields of materials science. They exhibit extraordinary strength and unique electrical properties, and are efficient conductors of heat. Inorganic nanotubes have also been synthesized.
Nanotubes are members of the fullerene structural family, which also includes buckyballs. Whereas buckyballs are spherical in shape, a nanotube is cylindrical, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is in the order of a few nanometers (approximately 1/50,000th of the width of a human hair), while they can be up to several millimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs).
The nature of the bonding of a nanotube is described by applied quantum chemistry, specifically, orbital hybridization. The chemical bonding of nanotubes are composed entirely of sp2 bonds, similar to those of graphite. This bonding structure, which is stronger than the sp3 bonds found in diamond, provides the molecules with their unique strength. Nanotubes naturally align themselves into "ropes" held together by Van der Waals forces. Under high pressure, nanotubes can merge together, trading some sp² bonds for sp³ bonds, giving great possibility for producing strong, unlimited-length wires through high-pressure nanotube linking.[1]
Discovery
A 2006 editorial written by Marc Monthioux and Vladimir Kuznetsov in the journal Carbon has described the interesting and often misstated origin of the carbon nanotube. A large percentage of academic and popular literature attributes the discovery of hollow, nanometer sized tubes composed of graphitic carbon to Sumio Iijima of NEC in 1991.[2]
In 1952 Radushkevich and Lukyanovich published clear images of 50 nanometer diameter tubes made of carbon in the Soviet Journal of Physical Chemistry.[3] This discovery was largely unnoticed, the article was published in the Russian language, and Western scientists' access to Soviet press was limited during the Cold War. It is likely that carbon nanotubes were produced before this date, but the invention of the transmission electron microscope allowed the direct visualization of these structures.
Carbon nanotubes have been produced and observed under a variety of conditions prior to 1991. A paper by Oberlin, Endo, and Koyama published in 1976 clearly showed hollow carbon fibres with nanometer-scale diameters using a vapour-growth technique.[4] Additionally, the authors show a TEM image of a nanotube consisting of a single wall of graphene. Later, Endo has referred to this image as a single-walled nanotube.[5]
Furthermore, in 1979, John Abrahamson presented evidence of carbon nanotubes at the 14th Biennial Conference of Carbon at Penn State University. The conference paper described carbon nanotubes as carbon fibers which were produced on carbon anodes during arc discharge. A characterization of these fibres was given as well as hypotheses for their growth in a nitrogen atmosphere at low pressures.[6]
In 1981 a group of Soviet scientists published the results of chemical and structural characterization of carbon nanoparticles produced by a thermocatalytical disproportionation of carbon monoxide. Using TEM images and XRD patterns, the authors suggested that their “Carbon multi-layer tubular crystals” were formed by rolling graphene layers into cylinders. Additionally, they speculated that during rolling graphene layers into a cylinder, many different arrangements of graphene hexagonal nets are possible. They suggested two possibilities of such arrangements: circular arrangement (armchair nanotube) and a spiral, helical arrangement (chiral tube).[7]
In 1987, Howard G. Tennent of Hyperion Catalysis was issued a U.S. patent for the production of "cylindrical discrete carbon fibrils" with a "constant diameter between about 3.5 and about 70 nanometers…, length 10² times the diameter, and an outer region of multiple essentially continuous layers of ordered carbon atoms and a distinct inner core…."[8]
Iijima's discovery of carbon nanotubes in the insoluble material of arc-burned graphite rods[9] created the buzz that is now associated with carbon nanotubes. Nanotube research accelerated greatly following the independent discoveries[10][11] by Bethune at IBM[12] and Iijima at NEC of single-walled carbon nanotubes and methods to specifically produce them by adding transition-metal catalysts to the carbon in an arc discharge. The arc discharge technique was well-known to produce the famed Buckminster fullerene on a preparative scale,[13] and these results appeared to extend the run of accidental discoveries relating to fullerenes. The original observation of fullerenes in mass spectrometry was not anticipated,[14] and the first mass-production technique by Krätschmer and Huffman was used for several years before realising that it produced fullerenes.[13]
The discovery of nanotubes remains a contentious issue, especially because several scientists involved in the research could be likely candidates for the Nobel Prize. Many believe that Iijima's report in 1991 is of particular importance because it brought carbon nanotubes into the awareness of the scientific community as a whole. See the reference for a review of the history of the discovery of carbon nanotubes.[2]
Types of carbon nanotubes and related structures
Single-walled
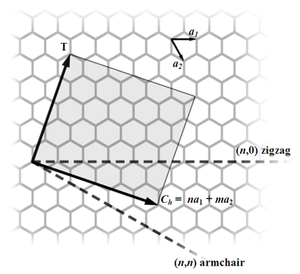
Most single-walled nanotubes (SWNT) have a diameter of close to 1 nanometer, with a tube length that can be many thousands of times longer. The structure of a SWNT can be conceptualized by wrapping a one-atom-thick layer of graphite called graphene into a seamless cylinder. The way the graphene sheet is wrapped is represented by a pair of indices (n,m) called the chiral vector. The integers n and m denote the number of unit vectors along two directions in the honeycomb crystal lattice of graphene. If m=0, the nanotubes are called "zigzag". If n=m, the nanotubes are called "armchair". Otherwise, they are called "chiral".
Single-walled nanotubes are a very important variety of carbon nanotube because they exhibit important electric properties that are not shared by the multi-walled carbon nanotube (MWNT) variants. Single-walled nanotubes are the most likely candidate for miniaturizing electronics beyond the micro electromechanical scale that is currently the basis of modern electronics. The most basic building block of these systems is the electric wire, and SWNTs can be excellent conductors.[15] One useful application of SWNTs is in the development of the first intramolecular field effect transistors (FETs). The production of the first intramolecular logic gate using SWNT FETs has recently become possible as well.[16] To create a logic gate you must have both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to oxygen and n-FETs when unexposed to oxygen, it is possible to protect half of a SWNT from oxygen exposure, while exposing the other half to oxygen. This results in a single SWNT that acted as a NOT logic gate with both p and n-type FETs within the same molecule.
Single-walled nanotubes are still very expensive to produce, around $1500 per gram as of 2000, and the development of more affordable synthesis techniques is vital to the future of carbon nanotechnology. If cheaper means of synthesis cannot be discovered, it would make it financially impossible to apply this technology to commercial-scale applications.[17] Several suppliers offer as-produced arc discharge SWNTs for ~$50–100 per gram as of 2007.[18][19]
Multi-walled
Multi-walled nanotubes(MWNT) consist of multiple layers of graphite rolled in on themselves to form a tube shape. There are two models which can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, e.g. a (0,8) single-walled nanotube (SWNT) within a larger (0,10) single-walled nanotube. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled up newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 3.3 Å. The special place of double-walled Carbon Nanotubes (DWNT) must be emphasized here because they combine very similar morphology and properties as compared to SWNT, while improving significantly their resistance to chemicals. This is especially important when functionalisation is required (this means grafting of chemical functions at the surface of the nanotubes) to add new properties to the CNT. In the case of SWNT, covalent functionalisation will break some C=C double bonds, leaving "holes" in the structure on the nanotube and thus modifying both its mechanical and electrical properties. In the case of DWNT, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003[20] by the CCVD technique, from the selective reduction of oxides solid solutions in methane and hydrogen.
Fullerite
Fullerites are the solid-state manifestation of fullerenes and related compounds and materials. Being highly incompressible nanotube forms, polymerized single-walled nanotubes (P-SWNT) are a class of fullerites and are comparable to diamond in terms of hardness. However, due to the way that nanotubes intertwine, P-SWNTs don't have the corresponding crystal lattice that makes it possible to cut diamonds neatly. This same structure results in a less brittle material, as any impact that the structure sustains is spread out throughout the material.
Torus
A nanotorus is a theoretically described carbon nanotube bent into a torus (doughnut shape). Nanotori have many unique properties, such as magnetic moments 1000 times larger than previously expected for certain specific radii.[21] Properties such as magnetic moment, thermal stability, etc. vary widely depending on radius of the torus and radius of the tube.[21][22]
Nanobud
Carbon NanoBuds are a newly discovered material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes. In this new material fullerene-like "buds" are covalently bonded to the outer sidewalls of the underlying carbon nanotube. This hybrid material has useful properties of both fullerenes and carbon nanotubes. In particular, they have been found to be exceptionally good field emitters.
Properties
Strength
Carbon nanotubes are one of the strongest and stiffest materials known, in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp² bonds formed between the individual carbon atoms. In 2000, a multi-walled carbon nanotube was tested to have a tensile strength of 63 GPa Since carbon nanotubes have a low density for a solid of 1.3-1.4 g/cm³,[17] its specific strength of up to 48,462 kN·m/kg is the best of known materials, compared to high-carbon steel's 154 kN·m/kg.
Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% and can increase the maximum strain the tube undergoes before fracture by releasing strain energy.
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional or bending stress.
Kinetic
Multi-walled nanotubes, multiple concentric nanotubes precisely nested within one another, exhibit a striking telescoping property whereby an inner nanotube core may slide, almost without friction, within its outer nanotube shell thus creating an atomically perfect linear or rotational bearing This is one of the first true examples of molecular nanotechnology, the precise positioning of atoms to create useful machines. Already this property has been utilized to create the world's smallest rotational motor. Future applications such as a gigahertz mechanical oscillator are also envisaged.
Electrical
Because of the symmetry and unique electronic structure of graphene, the structure of a nanotube strongly affects its electrical properties. For a given (n,m) nanotube, if n − m is a multiple of 3, then the nanotube is metallic, otherwise the nanotube is a semiconductor. Thus all armchair (n=m) nanotubes are metallic, and nanotubes (5,0), (6,4), (9,1), etc. are semiconducting. In theory, metallic nanotubes can have an electrical current density more than 1,000 times greater than metals such as silver and copper.
Thermal
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction," but good insulators laterally to the tube axis. It is predicted that carbon nanotubes will be able to transmit up to 6000 watts per meter per kelvin at room temperature; compare this to copper, a metal well-known for its good thermal conductivity, which only transmits 385 W/m/K. The temperature stability of carbon nanotubes is estimated to be up to 2800 degrees Celsius in vacuum and about 750 degrees Celsius in air.
Defects
As with any material, the existence of defects affects the material properties. Defects can occur in the form of atomic vacancies. High levels of such defects can lower the tensile strength by up to 85%. Another form of defect that may occur in carbon nanotubes is known as the Stone Wales defect, which creates a pentagon and heptagon pair by rearrangement of the bonds. Because of the very small structure of CNTs, the tensile strength of the tube is dependent on the weakest segment of it in a similar manner to a chain, where a defect in a single link diminishes the strength of the entire chain.
The tube's electrical properties are also affected by the presence of defects. A common result is the lowered conductivity through the defective region of the tube. Some defect formation in armchair-type tubes (which can conduct electricity) can cause the region surrounding that defect to become semiconducting. Furthermore single monoatomic vacancies induce magnetic properties.
The tube's thermal properties are heavily affected by defects. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path, and reduces the thermal conductivity of nanotube structures.
One-Dimensional Transport
Due to their nanoscale dimensions, electron transport in carbon nanotubes will take place through quantum effects and will only propagate along the axis of the tube.Because of this special transport property, carbon nanotubes are frequently referred to as “one-dimensional” in scientific articles.
Toxicity
Determining the toxicity of carbon nanotubes has been one of the most pressing questions in Nanotechnology. Results from various scientific tests on cells have so far proven confusing, with some results indicating it to be highly toxic and others showing no signs of toxicity.[23] This is primarily because of difficulties arising in spotting the nanotubes entering the cells from other carbon-based cell structures such as membranes. A recent research led by Alexandra Porter from the University of Cambridge shows once they are inside the cell, they accumulate in the cytoplasm and cause cell death.[24]
Synthesis
Techniques have been developed to produce nanotubes in sizeable quantities, including arc discharge, laser ablation, high pressure carbon monoxide (HiPCO), and chemical vapor deposition (CVD). Most of these processes take place in vacuum or with process gases. CVD growth of CNTs can take place in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth processes are making CNTs more commercially viable.
It is now thought by some that the catalysts or methods involved in forging damascus steel (a forging technique lost to time) may provide vital hints for manufacturing nanotubes cheaply, after they were recently discovered to be a component of that ancient sword metal.[25][26]
Arc discharge
Nanotubes were observed in 1991 in the carbon soot of graphite electrodes during an arc discharge, by using a current of 100 amps, that was intended to produce fullerenes.[27] However the first macroscopic production of carbon nanotubes was made in 1992 by two researchers at NEC's Fundamental Research Laboratory.[28] The method used was the same as in 1991. During this process, the carbon contained in the negative electrode sublimates because of the high temperatures caused by the discharge. Because nanotubes were initially discovered using this technique, it has been the most widely used method of nanotube synthesis.
The yield for this method is up to 30 percent by weight and it produces both single- and multi-walled nanotubes with lengths of up to 50 microns.[17]
Laser ablation
In the laser ablation process, a pulsed laser vaporizes a graphite target in a high temperature reactor while an inert gas is bled into the chamber. The nanotubes develop on the cooler surfaces of the reactor, as the vaporized carbon condenses. A water-cooled surface may be included in the system to collect the nanotubes.
It was invented by Richard Smalley and co-workers at Rice University, who at the time of the discovery of carbon nanotubes, were blasting metals with the laser to produce various metal molecules. When they heard of the discovery they substituted the metals with graphite to create multi-walled carbon nanotubes.[29] Later that year the team used a composite of graphite and metal catalyst particles (the best yield was from a cobalt and nickel mixture) to synthesise single-walled carbon nanotubes.[30]
This method has a yield of around 70% and produces primarily single-walled carbon nanotubes with a controllable diameter determined by the reaction temperature. However, it is more expensive than either arc discharge or chemical vapor deposition.[17]
Chemical vapor deposition (CVD)

The catalytic vapor phase deposition of carbon was first reported in 1959,[31] but it was not until 1993[32] that carbon nanotubes could be formed by this process. In 2007, researchers at the University of Cincinnati (UC) developed a process to grow 18 mm long aligned carbon nanotube arrays.[33]
During CVD, a substrate is prepared with a layer of metal catalyst particles, most commonly nickel, cobalt, iron, or a combination. The metal nanoparticles can also be produced by other ways, including reduction of oxides or oxides solid solutions. The diameters of the nanotubes that are to be grown are related to the size of the metal particles. This can be controlled by patterned (or masked) deposition of the metal, annealing, or by plasma etching of a metal layer. The substrate is heated to approximately 700°C. To initiate the growth of nanotubes, two gases are bled into the reactor: a process gas (such as ammonia, nitrogen, hydrogen, etc.) and a carbon-containing gas (such as acetylene, ethylene, ethanol, methane, etc.). Nanotubes grow at the sites of the metal catalyst; the carbon-containing gas is broken apart at the surface of the catalyst particle, and the carbon is transported to the edges of the particle, where it forms the nanotubes. This mechanism is still under discussion. The catalyst particles can stay at the tips of the growing nanotube during the growth process, or remain at the nanotube base, depending on the adhesion between the catalyst particle and the substrate.
CVD is a common method for the commercial production of carbon nanotubes. For this purpose, the metal nanoparticles will be carefully mixed with a catalyst support (e.g., MgO, Al2O3, etc) to increase the specific surface area for higher yield of the catalytic reaction of the carbon feedstock with the metal particles. One issue in this synthesis route is the removal of the catalyst support via an acid treatment, which sometimes could destroy the original structure of the carbon nanotubes. However, alternative catalyst supports that are soluble in water have been shown to be effective for nanotube growth.[34]
If a plasma is generated by the application of a strong electric field during the growth process (plasma enhanced chemical vapor deposition), then the nanotube growth will follow the direction of the electric field.[35] By properly adjusting the geometry of the reactor it is possible to synthesize vertically aligned carbon nanotubes[1](i.e., perpendicular to the substrate), a morphology that has been of interest to researchers interested in the electron emission from nanotubes. Without the plasma, the resulting nanotubes are often randomly oriented, resembling a bowl of spaghetti. Under certain reaction conditions, even in the absence of a plasma, closely spaced nanotubes will maintain a vertical growth direction resulting in a dense array of tubes resembling a carpet or forest.
Of the various means for nanotube synthesis, CVD shows the most promise for industrial scale deposition in terms of its price/unit ratio. There are additional advantages to the CVD synthesis of nanotubes. Unlike the above methods, CVD is capable of growing nanotubes directly on a desired substrate, whereas the nanotubes must be collected in the other growth techniques. The growth sites are controllable by careful deposition of the catalyst. Additionally, no other growth methods have been developed to produce vertically aligned nanotubes.[17] In 2007, a team from Meijo University has shown a high-efficiency CVD technique for growing carbon nanotubes from camphor.[36] A team of researchers at Rice University, until recently led by the late Dr. Richard Smalley, has concentrated upon finding methods to produce large, pure amounts of particular types of nanotubes. Their approach grows long fibers from many small seeds cut from a single nanotube; all of the resulting fibers were found to be of the same diameter as the original nanotube and are expected to be of the same type as the original nanotube. Further characterization of the resulting nanotubes and improvements in yield and length of grown tubes are needed.[37]
CVD growth of multi-walled nanotubes is used by several companies to produce materials on the tonne scale, including NanoLab, Bayer, Arkema, Nanocyl, Nanothinx , Hyperion Catalysis, Mitsui, and Showa Denko.
Natural, incidental, and controlled flame environments
Fullerenes and carbon nanotubes are not necessarily products of high-tech laboratories; they are commonly formed in such mundane places as ordinary flames,[38] produced by burning methane,[39] ethylene,[40] and benzene,[41] and they have been found in soot from both indoor and outdoor air.[42] However, these naturally occurring varieties can be highly irregular in size and quality because the environment in which they are produced is often highly uncontrolled. Thus, although they can be used in some applications, they can lack in the high degree of uniformity necessary to meet many needs of both research and industry. Recent efforts have focused on producing more uniform carbon nanotubes in controlled flame environments.[43][44][45][46] Nano-C, Inc of Westwood, Massachusetts, is producing flame synthesized single-walled carbon nanotubes. This method has promise for large scale, low cost nanotube synthesis, though it must compete with rapidly developing large scale CVD production.
Potential and current applications
- See also, for last current applications: Timeline of carbon nanotubes
The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering. The highest tensile strength an individual multi-walled carbon nanotube has been tested to be is 63 GPa.[47] Bulk nanotube materials may never achieve a tensile strength similar to that of individual tubes, but such composites may nevertheless yield strengths sufficient for many applications. Carbon nanotubes have already been used as composite fibers in polymers to improve the mechanical, thermal and electrical properties of the bulk product. A 2006 study published in Nature determined that some carbon nanotubes are present in damascus steel, possibly helping to account for the legendary strength of the (almost ancient) swords made of it.[48][49]
Structural
Because of the great mechanical properties of the carbon nanotubule, a variety of structures has been proposed ranging from everyday items like clothes and sports gear to combat jackets and space elevators.[50] However, the space elevator will require further efforts in refining carbon nanotube technology, as the practical tensile strength of carbon nanotubes can still be greatly improved.[17]
For perspective, outstanding breakthroughs have already been made. Pioneering work lead by Ray H. Baughman at the NanoTech Institute has shown that single and multi-walled nanotubes can produce materials with toughness un-matched in the man-made and natural worlds.[51] [52]
Recent research by James D. Iverson and Brad C. Edwards has revealed the possibility of cross-linking CNT molecules prior to incorporation in a polymer matrix to form a super high strength composite supermaterial. This CNT composite will have a tensile strength on the order of 20 million psi (138 GPa, for 106 MN·m/kg), revolutionizing many aspects of engineering design where low weight and high strength is required.[citation needed]
In electrical circuits
Carbon nanotubes have many properties—from their unique dimensions to an unusual current conduction mechanism—that make them ideal components of electrical circuits.
Nanotube based transistors have been made that operate at room temperature and that are capable of digital switching using a single electron.[53]
One major obstacle to realization of nanotubes has been the lack of technology for mass production. However, in 2001 IBM researchers demonstrated how nanotube transistors can be grown in bulk, not very differently from silicon transistors. The process they used is called "constructive destruction" which includes the automatic destruction of defective nanotubes on the wafer.[54]
This has since then been developed further and single-chip wafers with over ten billion correctly aligned nanotube junctions have been created. In addition it has been demonstrated that incorrectly aligned nanotubes can be removed automatically using standard lithography equipment.[55]
The first nanotube made integrated memory circuit was made in 2004. One of the main challenges has been regulating the conductivity of nanotubes. Depending on subtle surface features a nanotube may act as a plain conductor or as a semiconductor. A fully automated method has however been developed to remove non-semiconductor tubes.[56]
Other applications
Carbon nanotubes have also been implemented in nanoelectromechanical systems, including mechanical memory elements (NRAM being developed by Nantero Inc.) and nanoscale electric motors (see Nanomotor).
Carbon nanotubes have also been proposed as a possible gene delivery vehicle and for use in combination with radiofrequency fields to destroy cancer cells. [57] [58]
Eikos Inc of Franklin, Massachusetts and Unidym Inc. of Silicon Valley, California are developing transparent, electrically conductive films of carbon nanotubes to replace indium tin oxide (ITO). Carbon nanotube films are substantially more mechanically robust than ITO films, making them ideal for high reliability touch screens and flexible displays. Printable water-based inks of carbon nanotubes are desired to enable the production of these films to replace ITO.[59] Nanotube films show promise for use in displays for computers, cell phones, PDAs, and ATMs.
CNT molecular modeling software
Molecular models of carbon nanotubes
References
- ^ Yildirim, T. (2000). "Pressure-induced interlinking of carbon nanotubes". Physical Review B. 62: 19.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Monthioux, Marc (2006). "Who should be given the credit for the discovery of carbon nanotubes?" (PDF). Carbon. 44. Retrieved 2007-07-26.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ [http://carbon.phys.msu.ru/publications/1952-radushkevich-lukyanovich.pdf Radushkevich-Lukyanovich (1952) in russian
- ^ Oberlin, A. (March 1976). "Filamentous growth of carbon through benzene decomposition". 32: 335–349. Retrieved 2007-07-28.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Endo, Morinobu; Dresselhaus, M. S. (October 26, 2002), Carbon Fibers and Carbon Nanotubes (Interview, Nagano, Japan) (PDF), retrieved July 26, 2007
- ^ Abrahamson, John; Wiles, Peter G.; Rhoades, Brian L. (1999), "Structure of Carbon Fibers Found on Carbon Arc Anodes", Carbon, 37 (11): 1873
- ^ Izvestiya Akademii Nauk SSSR, Metals. 1982, #3, p.12-17 [in Russian]
- ^ US 4663230, Tennent, Howard G., "Carbon fibrils, method for producing same and compositions containing same", issued 1987-05-05
- ^ Iijima, Sumio (1991). "Helical microtubules of graphitic carbon". Nature. 354: 56–58.
- ^ Bethune, D. S. (17 June 1993). "Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls". Nature. 363: 605–607. doi:10.1038/363605a0.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Iijama, Sumio (1993). "Single-shell carbon nanotubes of 1-nm diameter". Nature. 363: 603–605.
- ^ "The Discovery of Single-Wall Carbon Nanotubes at IBM". IBM. Retrieved 2007-07-26.
- ^ a b Krätschmer, W. (1990). "Solid C60: a new form of carbon". Nature. 347: 354–358.
- ^ Kroto, H. W. (1985). "C60: Buckminsterfullerene". Nature. 318: 162–163.
- ^ Dekker, Cees (May 1999). "Carbon nanotubes as molecular quantum wires" (PDF). Physics Today. 52 (5): 22–28. Retrieved 2007-07-28.
- ^ Martel, R. (December 2001). "Ambipolar Electrical Transport in Semiconducting Single-Wall Carbon Nanotubes". Physical Review Letters. 87 (25). Retrieved 2007-07-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f Collins, Philip G. (December 2000). "Nanotubes for Electronics" (PDF). Scientific American: 67, 68, and 69.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Carbon Solutions, Inc". Retrieved 2007-07-28.
- ^ "CarboLex". Retrieved 2007-07-28.
- ^ Flahaut, E. (2003). "Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes". Chemical Communications. 12: 1442–1443. Retrieved 2007-07-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Liu, Lei (2002). "Colossal Paramagnetic Moments in Metallic Carbon Nanotori". Physical Review Letters. 88 (21). Retrieved 2007-07-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Huhtala, Maria (2002). "Carbon nanotube structures: molecular dynamics simulation at realistic limit" (PDF). Computer Physics Communications. 146. Retrieved 2007-07-28.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Nano World: Nanotube toxicity exams differ". IBM. Retrieved 2007-11-16.
- ^ Porter, Alexandra (November, 2007). "Direct imaging of single-walled carbon nanotubes in cells". Nature Nanotechnology. 2 (11): 713–717.
{{cite journal}}: Check date values in:|date=(help) - ^ Inman, Mason (November 16, 2006). "Legendary Swords' Sharpness, Strength From Nanotubes, Study Says". National Geographic. Retrieved 2007-05-26.
- ^ "Secret's out for Saracen sabres". NewScientistTech. November 15, 2006.
- ^ Iijima, Sumio (1991). "Helical microtubules of graphitic carbon". Nature. 354: 56–58.
- ^ Ebbesen, T. W. (1992). "Large-scale synthesis of carbon nanotubes". Nature. 358: 220–222.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Guo, Ting (1995). "Self-Assembly of Tubular Fullerenes" (PDF). J. Phys. Chem. 99: 10694–10697.
- ^ Guo, Ting (1995). "Catalytic growth of single-walled manotubes by laser vaporization" (PDF). Chem. Phys. Lett. 243: 49–54.
- ^ Walker Jr., P. L. (1959). "Carbon Formation from Carbon Monoxide-Hydrogen Mixtures over Iron Catalysts.I. Properties of Carbon Formed" (PDF). J. Phys. Chem. 63: 133.
- ^ José-Yacamán, M. (1993). "Catalytic growth of carbon microtubules with fullerene structure". Appl. Phys. Lett. 62: 657.
- ^ Beckman, Wendy (April 27, 2007). "UC Researchers Shatter World Records with Length of Carbon Nanotube Arrays". University of Cincinnati.
- ^ Eftekhari, A. (2006). "High-yield synthesis of carbon nanotubes using a water-soluble catalyst support in catalytic chemical vapor deposition". Carbon. 44: 1343.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ren, Z. F. (1998). "Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass". Science. 282: 1105.
- ^ "Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology" (PDF). Journal of Physics. Retrieved 2007-08-15.
- ^ Boyd, Jade (November 17, 2006). "Rice chemists create, grow nanotube seeds". Rice University.
- ^ Singer, J.M. (1959). "Carbon formation in very rich hydrocarbon-air flames. I. Studies of chemical content, temperature, ionization and particulate matter". Seventh Symposium (International) on Combustion.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yuan, Liming (2001). "Nanotubes from methane flames". Chemical physics letters. 340: 237–241. doi:10.1016/S0009-2614(01)00435-3.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yuan, Liming (2001). "Ethylene flame synthesis of well-aligned multi-walled carbon nanotubes". Chemical physics letters. 346: 23–28. doi:10.1016/S0009-2614(01)00959-9.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Duan, H. M. (1994). "Nanoclusters Produced in Flames". Journal of Physical Chemistry. 98 (49): 12815–12818. doi:10.1021/j100100a001.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Murr, L. E. (2004). "Carbon nanotubes, nanocrystal forms, and complex nanoparticle aggregates in common fuel-gas combustion sources and the ambient air". Journal of Nanoparticle Research. 6: 241–251. doi:10.1023/B:NANO.0000034651.91325.40.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vander Wal, R.L. (2002). "Fe-catalyzed single-walled carbon nanotube synthesis within a flame environment". Combust. Flame. 130: 37–47.
- ^ Saveliev, A.V. (2003). "Metal catalyzed synthesis of carbon nanostructures in an opposed flow methane oxygen flame". Combust. Flame. 135: 27–33.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Height, M.J. (2004). "Flame synthesis of single-walled carbon nanotubes". Carbon. 42: 2295–2307.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Sen, S. (2004). "Flame synthesis of carbon nanofibres and nanofibre composites containing encapsulated metal particles". Nanotechnology. 15: 264–268.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Yu, Min-Feng (2000). "Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load". Science. 287: 637–640.
- ^ Inman, Mason (November 16, 2006). "Legendary Swords' Sharpness, Strength From Nanotubes, Study Says". National Geographic News.
- ^ "Secret's out for Saracen sabres". New Scientist. November 15, 2006.
- ^ Edwards, Brad C. (November 2003). The Space Elevator. BC Edwards. ISBN 0974651710.
- ^ Zhang, Mei (2005). "Strong, Transparent, Multifunctional, Carbon Nanotube Sheets". Science. 309 (5738): 1215–1219.
- ^ Dalton, Alan B. (2003). "Super-tough carbon-nanotube fibres". Nature. 423 (6941): 703.
- ^ Postma, Henk W. Ch. (2001). "Carbon Nanotube Single-Electron Transistors at Room Temperature". Science. 293 (5527).
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Collins, Philip G. (April 27, 2001). "Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown". Science. 292 (5517): 706–709.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Song, Jin (2004). "Scalable Interconnection and Integration of Nanowire Devices Without Registration". Nano Letters. 4 (5): 915–919.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Tesng, Yu-Chih (2004). "Monolithic Integration of Carbon Nanotube Devices with Silicon MOS Technology". Nano Letters. 4 (1): 123–127.
- ^ Singh, Ravi (2005). "Binding and condensation of plasmid DNA onto functionalized carbon nanotubes : Toward the construction of nanotube-based gene delivery vectors". J. Am. Chem. Soc. 127 (12): 4388–4396.
- ^ Gannon, Christopher J. (2007). "Carbon nanotube-enhanced thermal destruction of cancer cells in a noninvasive radiofrequency field". Cancer. Dec. 2007.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Simmons, Trevor (2007). "Large Area-Aligned Arrays from Direct Deposition of Single-Wall Carbon Nanotubes". J. Am. Chem. Soc. 129 (33): 10088–10089.
External links
This article's use of external links may not follow Wikipedia's policies or guidelines. |
- Carbon Nanotubes News
- New Scientist Special Report: a collection of nanotechnology articles, most on nanotubes
- Nanotube suppliers: International List of nanotubes suppliers
- The stuff of dreams, CNET
- The Nanotube site. Last updated 2007.07.08
- Nanotechnologies and nanotubes
- Animation of a (29,0) being struck by 10 sets of 9 Argon atoms at 10 eV each (opens in media player)
- The wonderous World of Carbon Nanotubes (In .pdf format, good introduction to nanotube)
- Nanowerk Nanotechnology Portal: Introduction to nanmoaterials and nanotubes
- nanotechweb.org nanotube and nanotechnology news and information
- Carbon - Super Stuff: Educational interactive with narration and 3D-models of nanotube, diamond, graphite and coal.
- Carbon nanotube on arxiv.org
- Untangling and Dispersing of Carbon Nanotubes using Ultrasonics
- Photoactive molecules inside nanotubes
- Carbon nanotech may have given swords of Damascus their edge, Nature 2006.
- Interdisciplinary student project giving an excellent overview of literature on synthesis and purification
- EU Marie Curie Network CARBIO: Multifunctional carbon nanotubes for biomedical applications
- Site on Single Walled Carbon Nanotube, physics and electronics of CNT-SWNT.
- Nanowire Computing Made Practical
