Pulmonary hypertension: Difference between revisions
m fixed CS1 errors: dates & General fixes using AWB (9816) |
Bluerasberry (talk | contribs) →Treatment: added information about a distinction between treatment strategies for different types of pulmonary hypertension... |
||
| (One intermediate revision by the same user not shown) | |||
| Line 91: | Line 91: | ||
==Treatment== |
==Treatment== |
||
Treatment is determined by whether the PH is arterial, venous, hypoxic, thromboembolic, or miscellaneous. Since pulmonary ''venous'' hypertension is synonymous with [[congestive heart failure]], the treatment is to optimize left ventricular function by the use of [[diuretic]]s, [[beta blocker]]s, [[ACE inhibitor]]s etc., or to repair/replace the [[mitral valve]] or [[aortic valve]]. |
Treatment is determined by whether the PH is arterial, venous, hypoxic, thromboembolic, or miscellaneous. Since pulmonary ''venous'' hypertension is synonymous with [[congestive heart failure]], the treatment is to optimize left ventricular function by the use of [[diuretic]]s, [[beta blocker]]s, [[ACE inhibitor]]s etc., or to repair/replace the [[mitral valve]] or [[aortic valve]]. |
||
Patients with left heart failure or [[Hypoxemia|hypoxemic]] lung diseases (groups II or III pulmonary hypertension) should not routinely be treated with vasoactive agents including prostanoids, phosphodiesterase inhibitors, or endothelin antagonists, as these are approved for the different condition called pulmonary arterial hypertension.<ref name="ACCPandATSfive">{{Citation |author1 = American College of Chest Physicians |author1-link = American College of Chest Physicians |author2 = American Thoracic Society |author2-link = American Thoracic Society |date = September 2013 |title = Five Things Physicians and Patients Should Question |publisher = American College of Chest Physicians and American Thoracic Society |work = [[Choosing Wisely]]: an initiative of the [[ABIM Foundation]] |page = |url = http://www.choosingwisely.org/doctor-patient-lists/american-college-of-chest-physicians-and-american-thoracic-society/ |accessdate = 6 January 2013}}, which cites |
|||
*{{Cite journal |
|||
| last1 = McLaughlin | first1 = V. V. |
|||
| last2 = Archer | first2 = S. L. |
|||
| last3 = Badesch | first3 = D. B. |
|||
| last4 = Barst | first4 = R. J. |
|||
| last5 = Farber | first5 = H. W. |
|||
| last6 = Lindner | first6 = J. R. |
|||
| last7 = Mathier | first7 = M. A. |
|||
| last8 = McGoon | first8 = M. D. |
|||
| last9 = Park | first9 = M. H. |
|||
| last10 = Rosenson | first10 = R. S. |
|||
| last11 = Rubin | first11 = L. J. |
|||
| last12 = Tapson | first12 = V. F. |
|||
| last13 = Varga | first13 = J. |
|||
| last14 = Harrington | first14 = R. A. |
|||
| last15 = Anderson | first15 = J. L. |
|||
| last16 = Bates | first16 = E. R. |
|||
| last17 = Bridges | first17 = C. R. |
|||
| last18 = Eisenberg | first18 = M. J. |
|||
| last19 = Ferrari | first19 = V. A. |
|||
| last20 = Grines | first20 = C. L. |
|||
| last21 = Hlatky | first21 = M. A. |
|||
| last22 = Jacobs | first22 = A. K. |
|||
| last23 = Kaul | first23 = S. |
|||
| last24 = Lichtenberg | first24 = R. C. |
|||
| last25 = Lindner | first25 = J. R. |
|||
| last26 = Moliterno | first26 = D. J. |
|||
| last27 = Mukherjee | first29 = R. S. | first28 = G. M. |
|||
| last29 = Rosenson |
|||
| last28 = Pohost |
|||
| doi = 10.1161/CIRCULATIONAHA.109.192230 |
|||
| last30 = Schofield |
|||
| title = ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in Collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association | first30 = R. S. | first27 = D. |
|||
| journal = Circulation |
|||
| volume = 119 |
|||
| issue = 16 |
|||
| pages = 2250–2294 |
|||
| year = 2009 |
|||
| pmid = 19332472 |
|||
| pmc = |
|||
| displayauthors = 30 |
|||
}} |
|||
*{{Cite journal |
|||
| last1 = Galie | first1 = N. |
|||
| last2 = Hoeper | first2 = M. M. |
|||
| last3 = Humbert | first3 = M. |
|||
| last4 = Torbicki | first4 = A. |
|||
| last5 = Vachiery | first5 = J. -L. |
|||
| last6 = Barbera | first6 = J. A. |
|||
| last7 = Beghetti | first7 = M. |
|||
| last8 = Corris | first8 = P. |
|||
| last9 = Gaine | first9 = S. |
|||
| last10 = Gibbs | first10 = J. S. |
|||
| last11 = Gomez-Sanchez | first11 = M. A. |
|||
| last12 = Jondeau | first12 = G. |
|||
| last13 = Klepetko | first13 = W. |
|||
| last14 = Opitz | first14 = C. |
|||
| last15 = Peacock | first15 = A. |
|||
| last16 = Rubin | first16 = L. |
|||
| last17 = Zellweger | first17 = M. |
|||
| last18 = Simonneau | first18 = G. |
|||
| last19 = Vahanian | first19 = A. |
|||
| last20 = Auricchio | first20 = A. |
|||
| last21 = Bax | first21 = J. |
|||
| last22 = Ceconi | first22 = C. |
|||
| last23 = Dean | first23 = V. |
|||
| last24 = Filippatos | first24 = G. |
|||
| last25 = Funck-Brentano | first25 = C. |
|||
| last26 = Hobbs | first26 = R. |
|||
| last27 = Kearney | first27 = P. |
|||
| last28 = McDonagh | first28 = T. |
|||
| last29 = McGregor | first29 = K. |
|||
| last30 = Popescu | first30 = B. A. |
|||
| author31 = ESC Committee for Practice Guidelines (CPG) |
|||
| title = Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) |
|||
| doi = 10.1093/eurheartj/ehp297 |
|||
| journal = European Heart Journal |
|||
| volume = 30 |
|||
| issue = 20 |
|||
| pages = 2493–2537 |
|||
| year = 2009 |
|||
| pmid = 19713419 |
|||
| pmc = |
|||
| displayauthors = 30 |
|||
}} |
|||
*{{Cite journal |
|||
| last1 = Hoeper | first1 = M. M. |
|||
| last2 = Barberà | first2 = J. A. |
|||
| last3 = Channick | first3 = R. N. |
|||
| last4 = Hassoun | first4 = P. M. |
|||
| last5 = Lang | first5 = I. M. |
|||
| last6 = Manes | first6 = A. |
|||
| last7 = Martinez | first7 = F. J. |
|||
| last8 = Naeije | first8 = R. |
|||
| last9 = Olschewski | first9 = H. |
|||
| last10 = Pepke-Zaba | first10 = J. |
|||
| last11 = Redfield | first11 = M. M. |
|||
| last12 = Robbins | first12 = I. M. |
|||
| last13 = Souza | first13 = R. R. |
|||
| last14 = Torbicki | first14 = A. |
|||
| last15 = McGoon | first15 = M. |
|||
| title = Diagnosis, Assessment, and Treatment of Non-Pulmonary Arterial Hypertension Pulmonary Hypertension |
|||
| doi = 10.1016/j.jacc.2009.04.008 |
|||
| journal = Journal of the American College of Cardiology |
|||
| volume = 54 |
|||
| issue = 1 Suppl |
|||
| pages = S85–S96 |
|||
| year = 2009 |
|||
| pmid = 19555862 |
|||
| pmc = |
|||
}}</ref> To make the distinction, doctors at a minimum will conduct [[cardiac catheterization]] of the right heart, echocardiography, chest CT, a six-minute walk test, and [[pulmonary function testing]].<ref name="ACCPandATSfive"/> Using treatments for other kinds of pulmonary hypertension in patients with these conditions can harm the patient and wastes substantial medical resources.<ref name="ACCPandATSfive"/> |
|||
In PAH, lifestyle changes, [[digoxin]], [[diuretic]]s, oral [[anticoagulant]]s, and oxygen therapy are considered ''conventional'' therapy, but have never been proven to be beneficial in a randomized, prospective manner.{{Citation needed|date=November 2009}} |
In PAH, lifestyle changes, [[digoxin]], [[diuretic]]s, oral [[anticoagulant]]s, and oxygen therapy are considered ''conventional'' therapy, but have never been proven to be beneficial in a randomized, prospective manner.{{Citation needed|date=November 2009}} |
||
Revision as of 15:40, 7 January 2014
| Pulmonary hypertension | |
|---|---|
| Specialty | Cardiology, pulmonology |
Pulmonary hypertension (PH) is an increase of blood pressure in the pulmonary artery, pulmonary vein, or pulmonary capillaries, together known as the lung vasculature, leading to shortness of breath, dizziness, fainting, leg swelling and other symptoms. Pulmonary hypertension can be a severe disease with a markedly decreased exercise tolerance and heart failure. It was first identified by Ernst von Romberg in 1891.[1] According to the most recent classification, it can be one of five different types: arterial, venous, hypoxic, thromboembolic or miscellaneous.[2]
Signs and symptoms
Because symptoms may develop very gradually, patients may delay seeing a physician for years. Common symptoms are shortness of breath, fatigue, non-productive cough, angina pectoris, fainting or syncope, peripheral edema (swelling around the ankles and feet), and rarely hemoptysis (coughing up blood).
Pulmonary venous hypertension typically presents with shortness of breath while lying flat or sleeping (orthopnea or paroxysmal nocturnal dyspnea), while pulmonary arterial hypertension (PAH) typically does not.
A detailed family history is established to determine whether the disease might be familial. A history of exposure to drugs such as cocaine, methamphetamine, alcohol leading to cirrhosis, and tobacco leading to emphysema are considered significant. A physical examination is performed to look for typical signs of pulmonary hypertension, including a loud S2 (pulmonic valve closure sound), (para)sternal heave, jugular venous distension, pedal edema, ascites, hepatojugular reflux, clubbing etc. Evidence of tricuspid insufficiency is also sought and, if present, is consistent with the presence of pulmonary hypertension.
Causes and classification
A 1973 meeting organized by the World Health Organization was the first to attempt classification of pulmonary hypertension. A distinction was made between primary and secondary PH, and primary PH was divided in the "arterial plexiform", "veno-occlusive" and "thromboembolic" forms.[3] A second conference in 1998 at Évian-les-Bains also addressed the causes of secondary PH (i.e. those due to other medical conditions),[4] and in 2003, the 3rd World Symposium on Pulmonary Arterial Hypertension was convened in Venice to modify the classification based on new understandings of disease mechanisms. The revised system developed by this group provides the current framework for understanding pulmonary hypertension.[2] The system includes several improvements over the former 1998 Evian Classification system. Risk factor descriptions were updated, and the classification of congenital systemic-to pulmonary shunts was revised. A new classification of genetic factors in PH was recommended, but not implemented because available data were judged to be inadequate.[2]
The Venice 2003 Revised Classification system can be summarized as follows:[2]
- WHO Group I - Pulmonary arterial hypertension (PAH)
- Idiopathic (IPAH)
- Familial (FPAH)
- Associated with other diseases (APAH): collagen vascular disease (e.g. scleroderma), congenital shunts between the systemic and pulmonary circulation, portal hypertension, HIV infection, drugs, toxins, or other diseases or disorders
- Associated with venous or capillary disease
- WHO Group II - Pulmonary hypertension associated with left heart disease
- Atrial or ventricular disease
- Valvular disease (e.g. mitral stenosis)
- WHO Group III - Pulmonary hypertension associated with lung diseases and/or hypoxemia
- Chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD)
- Sleep-disordered breathing, alveolar hypoventilation
- Chronic exposure to high altitude
- Developmental lung abnormalities
- WHO Group IV - Pulmonary hypertension due to chronic thrombotic and/or embolic disease
- Pulmonary embolism in the proximal or distal pulmonary arteries
- Embolization of other matter, such as tumor cells or parasites
- WHO Group V - Miscellaneous
The classification does not include sickle cell disease,[5] Human herpesvirus 8, also associated with Kaposi's sarcoma, has been demonstrated in patients with PAH, suggesting that this virus may play a role in its development.[6] Recent studies have been unable to find an association between human herpesvirus 8 and idiopathic pulmonary arterial hypertension.[citation needed]
Pathogenesis
Whatever the initial cause, pulmonary arterial hypertension (WHO Group I) involves the vasoconstriction or tightening of blood vessels connected to and within the lungs. This makes it harder for the heart to pump blood through the lungs, much as it is harder to make water flow through a narrow pipe as opposed to a wide one. Over time, the affected blood vessels become both stiffer and thicker, in a process known as fibrosis. This further increases the blood pressure within the lungs and impairs their blood flow. In addition, the increased workload of the heart causes hypertrophy of the right ventricle, making the heart less able to pump blood through the lungs, ultimately causing right heart failure (a condition known as cor pulmonale). As the blood flowing through the lungs decreases, the left side of the heart receives less blood. This blood may also carry less oxygen than normal. Therefore it becomes harder and harder for the left side of the heart to pump to supply sufficient oxygen to the rest of the body, especially during physical activity.
Pathogenesis in pulmonary venous hypertension (WHO Group II) is completely different. There is no obstruction to blood flow in the lungs. Instead, the left heart fails to pump blood efficiently, leading to pooling of blood in the lungs. This causes pulmonary edema and pleural effusions.
In hypoxic pulmonary hypertension (WHO Group III), the low levels of oxygen are thought to cause vasoconstriction or tightening of pulmonary arteries. This leads to a similar pathophysiology as pulmonary arterial hypertension.
In chronic thromboembolic pulmonary hypertension (WHO Group IV), the blood vessels are blocked or narrowed with blood clots. Again, this leads to a similar pathophysiology as pulmonary arterial hypertension.
Molecular pathology
The molecular mechanism of pulmonary arterial hypertension (PAH) is not known yet, but it is believed that the endothelial dysfunction results in a decrease in the synthesis of endothelium-derived vasodilators such as nitric oxide and prostacyclin. Moreover, there’s a stimulation of the synthesis of vasoconstrictors such as thromboxane and vascular endothelial growth factor (VEGF). These results in a severe vasoconstriction and smooth muscle and adventitial hypertrophy characteristic of patients with PAH.[7]
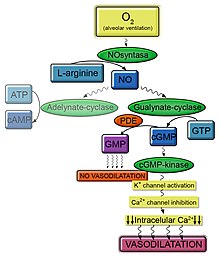
In normal conditions, the nitric oxide synthase produces nitric oxide from L-arginine in presence of oxygen. Adenylate-cyclase and gualynate-cyclase are activated in presence of nitric oxide and these enzymes produce cAMP and cGMP respectively. The cGMP is produced by a type of guanylate cyclase (which is a kind of pyrophosphate-liase cyclase): the soluble guanylate cyclase (or sGC), that catalyzes the formation of cGMP from GTP. sGC is a heterodimer made up of one α subunit and one β sub-unit in each chain. It also contains a prosthetic heme group, required for NO binding. The union of NO and sGC produces a conformational enzyme change that stimulates cGMP production.[8]
In the vascular endothelium, cGMP activates cGMP kinase or PKG (protein kinase G), which is an enzyme that belongs to a type of serine/threonine - specific protein kinase. PKG is a dimer composed of two similar polypeptides chains that share a common molecular structure. Each subunit contains a catalytic domain and regulatory domain. GMP-kinase activates potassium channels and subsequently the inhibition of calcium channels. Thus, this process leads to a reduction of intracellular calcium and finally a vasodilation.[9]
Phosphodiesterase type V (PDE5), which is abundant in the pulmonary tissue, is a metalohydrolase that hydrolyzes the cyclic bond of cGMP in the presence of divalent cations (Zn2+). Actually, Zn2+ union is necessary for PDE5 activity. In the N-terminal region (regulatory domain) of PDE5 there is an aminoacid sequence (residues 142-526) that joins cGMP. This sequence of PDE5 is divided in two domains; GAF-A and GAF-B; but only GAF-A has the necessary affinity to bind cGMP. This union increases the catalytic activity and it is stabilized by a close serine phosphorylation (performed by a kinase). Consequently, the concentration of cGMP decreases and the vasodilation is stopped.[8]
Patients with PAH produce less NO and others vasodilators and produce more vasoconstrictors. Consequently, this molecular pathway doesn’t work properly and it results in a constant vasoconstriction. For this reason, NO and PDE5 inhibitors such as tadalafil or sildenafil are possible therapies.[9] Tadalafil, for example, causes a vasodilation mediated by nitric oxide in the pulmonary endothelium.
Diagnosis

Because pulmonary hypertension can be of five major types, a series of tests must be performed to distinguish pulmonary arterial hypertension from venous, hypoxic, thromboembolic, or miscellaneous varieties.
Further procedures are required to confirm the presence of pulmonary hypertension and exclude other possible diagnoses. These generally include pulmonary function tests; blood tests to exclude HIV, autoimmune diseases, and liver disease; electrocardiography (ECG); arterial blood gas measurements; X-rays of the chest (followed by high-resolution CT scanning if interstitial lung disease is suspected); and ventilation-perfusion or V/Q scanning to exclude chronic thromboembolic pulmonary hypertension. Biopsy of the lung is usually not indicated unless the pulmonary hypertension is thought to be due to an underlying interstitial lung disease; further, lung biopsies are fraught with risks of bleeding due to the high intrapulmonary blood pressure. Clinical improvement is often measured by a "six-minute walk test", i.e. the distance a patient can walk in six minutes. Stability and improvement in this measurement correlate with better survival. Blood BNP level is also being used now to follow progress of patients with pulmonary hypertension. [citation needed]

Diagnosis of PAH requires the presence of pulmonary hypertension. Although pulmonary arterial pressure can be estimated on the basis of echocardiography, pressure measurements with a Swan-Ganz catheter through the right side of the heart provides the most definite assessment. PAOP (pulmonary artery occlusion pressure) and PVR (pulmonary vascular resistance) cannot be measured directly with echocardiography. Therefore diagnosis of PAH requires right-sided cardiac catheterization. A Swan-Ganz catheter can also measure the cardiac output, which is far more important in measuring disease severity than the pulmonary arterial pressure.
Normal pulmonary arterial pressure in a person living at sea level has a mean value of 12–16 mm Hg (1600–2100 Pa). Pulmonary hypertension is present when mean pulmonary artery pressure exceeds 25 mm Hg (3300 Pa) at rest or 30 mm Hg (4000 Pa) with exercise.
Mean pulmonary artery pressure (mPAP) should not be confused with systolic pulmonary artery pressure (sPAP), which is often reported on echocardiogram reports. A systolic pressure of 40 mm Hg typically implies a mean pressure of more than 25 mm Hg. Roughly, mPAP = 0.61•sPAP + 2.
Physical examination
A physical examination is performed to look for typical signs of pulmonary hypertension. These include altered heart sounds, such as a widely split S2 or second heart sound, a loud P2 or pulmonic valve closure sound (part of the second heart sound), (para)sternal heave, possible S3 or third heart sound, and pulmonary regurgitation. Other signs include an elevated jugular venous pressure, peripheral edema (swelling of the ankles and feet), ascites (abdominal swelling due to the accumulation of fluid), hepatojugular reflux, and clubbing.
Echocardiography
A meta-analysis of Doppler echocardiography for predicting right heart catheterization reported a sensitivity and specificity of 88% and 56%, respectively.[10]
Treatment
Treatment is determined by whether the PH is arterial, venous, hypoxic, thromboembolic, or miscellaneous. Since pulmonary venous hypertension is synonymous with congestive heart failure, the treatment is to optimize left ventricular function by the use of diuretics, beta blockers, ACE inhibitors etc., or to repair/replace the mitral valve or aortic valve.
Patients with left heart failure or hypoxemic lung diseases (groups II or III pulmonary hypertension) should not routinely be treated with vasoactive agents including prostanoids, phosphodiesterase inhibitors, or endothelin antagonists, as these are approved for the different condition called pulmonary arterial hypertension.[11] To make the distinction, doctors at a minimum will conduct cardiac catheterization of the right heart, echocardiography, chest CT, a six-minute walk test, and pulmonary function testing.[11] Using treatments for other kinds of pulmonary hypertension in patients with these conditions can harm the patient and wastes substantial medical resources.[11]
In PAH, lifestyle changes, digoxin, diuretics, oral anticoagulants, and oxygen therapy are considered conventional therapy, but have never been proven to be beneficial in a randomized, prospective manner.[citation needed]
High dose calcium channel blockers are useful in only 5% of IPAH patients who are vasoreactive by Swan-Ganz catheter. Unfortunately, calcium channel blockers have been largely misused, being prescribed to many patients with non-vasoreactive PAH, leading to excess morbidity and mortality. The criteria for vasoreactivity have changed. Only those patients whose mean pulmonary artery pressure falls by more than 10 mm Hg to less than 40 mm Hg with an unchanged or increased cardiac output when challenged with adenosine, epoprostenol, or nitric oxide are considered vasoreactive.[12] Of these, only half of the patients are responsive to calcium channel blockers in the long term.[13]
A number of agents have recently been introduced for primary and secondary PAH. The trials supporting the use of these agents have been relatively small, and the only measure consistently used to compare their effectivity is the "6 minute walk test". Many have no data on mortality benefit or time to progression.[14]
Vasoactive substances
Many pathways are involved in the abnormal proliferation and contraction of the smooth muscle cells of the pulmonary arteries in patients with pulmonary arterial hypertension. Three of these pathways are important since they have been targeted with drugs — endothelin receptor antagonists, phosphodiesterase type 5 (PDE-5) inhibitors, and prostacyclin derivatives.
Because inexpensive generic drugs for this disease are not widely available, the World Health Organization does not include them in its model list of essential medicines.
Prostaglandins
Prostacyclin (prostaglandin I2) is commonly considered the most effective treatment for PAH. Epoprostenol (synthetic prostacyclin, marketed as Flolan) is given via continuous infusion that requires a semi-permanent central venous catheter. This delivery system can cause sepsis and thrombosis. Prostacyclin is unstable, and therefore has to be kept on ice during administration. Since it has a half-life of 3 to 5 minutes, the infusion has to be continuous (24/7), and interruption can be fatal. Other prostanoids have therefore been developed. Treprostinil (Remodulin) can be given intravenously or subcutaneously, but the subcutaneous form can be very painful. An increased risk of sepsis with intravenous Remodulin has been reported by the CDC. Iloprost (Ilomedin) is also used in Europe intravenously and has a longer half life. Iloprost (marketed as Ventavis) was the only inhaled form of prostacyclin approved for use in the US and Europe, until the inhaled form of treprostinil was approved by the FDA in July 2009 and is marketed under the trade name Tyvaso. The inhaled form of administration has the advantage of selective deposition in the lungs with less systemic side effects, however coughing and throat irritation commonly occur. Oral and inhaled forms of Remodulin are under development. Beraprost is an oral prostanoid available in South Korea and Japan.
Endothelin receptor antagonists
The dual (ETA and ETB) endothelin receptor antagonist bosentan (marketed as Tracleer) was approved in 2001. Sitaxentan (Thelin), a selective endothelin receptor antagonist that blocks only the action of ETA, was approved for use in Canada, Australia, and the European Union.[15] but not in the United States. In 2010, Pfizer withdrew Thelin worldwide because of fatal liver complications. A similar drug, ambrisentan is marketed as Letairis in U.S. by Gilead Sciences.[16] In addition, another dual/nonselective endothelin antagonist, Actelion-1, from the makers of Tracleer, will enter clinical trials in 2008.
Phosphodiesterase type 5 inhibitors
The U.S. FDA approved Sildenafil, a selective inhibitor of cGMP specific phosphodiesterase type 5 (PDE5), for the treatment of PAH in 2005. It is marketed for PAH as Revatio. In 2009, they also approved Tadalafil, another PDE5 inhibitor, marketed under the name Adcirca.[17] PDE5 inhibitors are believed to increase pulmonary artery vasodilation, and inhibit vascular remodeling, thus lowering pulmonary arterial pressure and pulmonary vascular resistance.[citation needed]
Tadalafil is taken orally, as well as sildenafil, and it is rapidly absorbed (serum levels are detectable at 20 minutes). The recommended dose is 40 mg in one single dose per day and the T1/2 (biological half-life) hovers around 17.5 hours in healthy subjects.[18] Moreover, if we consider pharmacoeconomic implications, patients that take tadalafil would pay ⅔ of the cost of sildenafil therapy.[19] However, there are some adverse effects of this drug such as headache, diarrhea, nausea, back pain, dyspepsia, flushing and myalgia.[20]
Activators of soluble guanylate cyclase
Soluble guanylate cyclase (sGC) is the intracellular receptor for NO. As of April 2009[update], the sGC activators cinaciguat and riociguat were undergoing clinical trials for the treatment of PAH. In October 2013, riociguat (Adempas), was FDA approved for the treatment of PAH. It is the first of its class.
Surgical
Atrial septostomy is a surgical procedure that creates a communication between the right and left atria. It relieves pressure on the right side of the heart, but at the cost of lower oxygen levels in blood (hypoxia).
Lung transplantation cures pulmonary arterial hypertension, but leaves the patient with the complications of transplantation, and a post-surgical median survival of just over five years.[21]
Pulmonary thromboendarterectomy (PTE) is a surgical procedure that is used for chronic thromboembolic pulmonary hypertension. It is the surgical removal of an organized thrombus (clot) along with the lining of the pulmonary artery; it is a very difficult, major procedure that is currently performed in a few select centers. Case series show remarkable success in most patients.[citation needed]
Treatment regimens for hypoxic and miscellaneous varieties of pulmonary hypertension have not been established. However, studies of several agents are currently enrolling patients. Many physicians will treat these diseases with the same medications as for PAH, until better options become available. Such treatment is called off-label use.
Monitoring
Patients are normally monitored through commonly available tests such as:
- pulse oximetry
- arterial blood gas tests
- chest X-rays
- serial ECG tests
- serial echocardiography
- spirometry or more advanced lung function studies
Prognosis
The NIH IPAH registry from the 1980s showed an untreated median survival of 2–3 years from time of diagnosis, with the cause of death usually being right ventricular failure (cor pulmonale).[citation needed] A recent outcome study of those patients who had started treatment with bosentan (Tracleer) showed that 89% patients were alive at 2 years.[22] With new therapies, survival rates are increasing.[23] For 2,635 patients enrolled in The Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL Registry) from March 2006 to December 2009, 1-, 3-, 5-, and 7-year survival rates were 85%, 68%, 57%, and 49%, respectively. For patients with idiopathic/familial PAH, survival rates were 91%, 74%, 65%, and 59%.[24]
Levels of mortality are very high in pregnant women with severe pulmonary hypertension.[25] Pregnancy is sometimes described as contraindicated in these women.[26][27][28][29]
Epidemiology
Idiopathic pulmonary arterial hypertension is a rare disease with an incidence of about 2-3 per million per year[30] and a prevalence of about 15 per million. Adult females are almost three times as likely to present with IPAH than adult males. The presentation of IPAH within children is more evenly split along gender lines.
Other forms of PAH are far more common. In scleroderma the incidence has been estimated to be 6 to 60% of all patients, in rheumatoid arthritis up to 21%, in systemic lupus erythematosus 4 to 14%, in portal hypertension between 2 to 5%, in HIV about 0.5%, and in sickle cell disease ranging from 20 to 40%.
Diet pills such as Fen-Phen produced an annual incidence of 25-50 per million per year.
Pulmonary venous hypertension is exceedingly common, since it occurs in most patients symptomatic with congestive heart failure.
Up to 4% of people who suffer a pulmonary embolism go on to develop chronic thromboembolic disease including pulmonary hypertension.
Only about 1.1% of patients with COPD develop pulmonary hypertension with no other disease to explain the high pressure. Sleep apnea is usually associated with only very mild pulmonary hypertension, typically below the level of detection. On the other hand Pickwickian syndrome (obesity-hypoventilation syndrome) is very commonly associated with right heart failure due to pulmonary hypertension.
See also
References
- ^ von Romberg, Ernst (1891–1892). "Über Sklerose der Lungenarterie". Dtsch Arch Klin Med (in German). 48: 197–206.
{{cite journal}}: CS1 maint: date format (link) - ^ a b c d Simonneau G, Galiè N, Rubin LJ; et al. (2004). "Clinical classification of pulmonary hypertension". J. Am. Coll. Cardiol. 43 (12 Suppl S): 5S–12S. doi:10.1016/j.jacc.2004.02.037. PMID 15194173.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hatano S, Strasser R (1975). Primary pulmonary hypertension. Geneva: World Health Organization.
- ^ Rich S, Rubin LJ, Abenhail L; et al. (1998). Executive summary from the World Symposium on Primary Pulmonary Hypertension (Evian, France, September 6–10, 1998). Geneva: The World Health Organization. Archived from the original on April 8, 2002.
{{cite book}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Gladwin MT, Sachdev V, Jison ML; et al. (2004). "Pulmonary hypertension as a risk factor for death in patients with sickle cell disease". N. Engl. J. Med. 350 (9): 886–95. doi:10.1056/NEJMoa035477. PMID 14985486.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Cool CD, Rai PR, Yeager ME; et al. (2003). "Expression of human herpesvirus 8 in primary pulmonary hypertension". N. Engl. J. Med. 349 (12): 1113–22. doi:10.1056/NEJMoa035115. PMID 13679525.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Budhiraja R, Tuder RM, Hassoun. PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109:159–165.
- ^ a b Fosfodiesterasas del AMPc y del GMPc en el cerebro: Expresión en procesos neuroinflamatorios y neurodegenerativos. URL: http://www.tesisenred.net/bitstream/handle/10803/891/03.ERI_METODOS.pdf?sequence=4. Viewed 3 November 2012.
- ^ a b Ghofrani HA, Pepke-Zaba J, Barbera JA, et al. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:68S–72S.
- ^ Taleb M, Khuder S, Tinkel J, Khouri SJ (2013). "The diagnostic accuracy of Doppler echocardiography in assessment of pulmonary artery systolic pressure: a meta-analysis". Echocardiography. 30 (3): 258–65. doi:10.1111/echo.12061. PMID 23227919.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c American College of Chest Physicians; American Thoracic Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Chest Physicians and American Thoracic Society, retrieved 6 January 2013, which cites
- McLaughlin, V. V.; Archer, S. L.; Badesch, D. B.; Barst, R. J.; Farber, H. W.; Lindner, J. R.; Mathier, M. A.; McGoon, M. D.; Park, M. H.; Rosenson, R. S.; Rubin, L. J.; Tapson, V. F.; Varga, J.; Harrington, R. A.; Anderson, J. L.; Bates, E. R.; Bridges, C. R.; Eisenberg, M. J.; Ferrari, V. A.; Grines, C. L.; Hlatky, M. A.; Jacobs, A. K.; Kaul, S.; Lichtenberg, R. C.; Lindner, J. R.; Moliterno, D. J.; Mukherjee, D.; Pohost, G. M.; Rosenson, R. S.; Schofield, R. S. (2009). "ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in Collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association". Circulation. 119 (16): 2250–2294. doi:10.1161/CIRCULATIONAHA.109.192230. PMID 19332472.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Galie, N.; Hoeper, M. M.; Humbert, M.; Torbicki, A.; Vachiery, J. -L.; Barbera, J. A.; Beghetti, M.; Corris, P.; Gaine, S.; Gibbs, J. S.; Gomez-Sanchez, M. A.; Jondeau, G.; Klepetko, W.; Opitz, C.; Peacock, A.; Rubin, L.; Zellweger, M.; Simonneau, G.; Vahanian, A.; Auricchio, A.; Bax, J.; Ceconi, C.; Dean, V.; Filippatos, G.; Funck-Brentano, C.; Hobbs, R.; Kearney, P.; McDonagh, T.; McGregor, K.; Popescu, B. A.; ESC Committee for Practice Guidelines (CPG) (2009). "Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT)". European Heart Journal. 30 (20): 2493–2537. doi:10.1093/eurheartj/ehp297. PMID 19713419.
{{cite journal}}: Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Hoeper, M. M.; Barberà, J. A.; Channick, R. N.; Hassoun, P. M.; Lang, I. M.; Manes, A.; Martinez, F. J.; Naeije, R.; Olschewski, H.; Pepke-Zaba, J.; Redfield, M. M.; Robbins, I. M.; Souza, R. R.; Torbicki, A.; McGoon, M. (2009). "Diagnosis, Assessment, and Treatment of Non-Pulmonary Arterial Hypertension Pulmonary Hypertension". Journal of the American College of Cardiology. 54 (1 Suppl): S85–S96. doi:10.1016/j.jacc.2009.04.008. PMID 19555862.
- McLaughlin, V. V.; Archer, S. L.; Badesch, D. B.; Barst, R. J.; Farber, H. W.; Lindner, J. R.; Mathier, M. A.; McGoon, M. D.; Park, M. H.; Rosenson, R. S.; Rubin, L. J.; Tapson, V. F.; Varga, J.; Harrington, R. A.; Anderson, J. L.; Bates, E. R.; Bridges, C. R.; Eisenberg, M. J.; Ferrari, V. A.; Grines, C. L.; Hlatky, M. A.; Jacobs, A. K.; Kaul, S.; Lichtenberg, R. C.; Lindner, J. R.; Moliterno, D. J.; Mukherjee, D.; Pohost, G. M.; Rosenson, R. S.; Schofield, R. S. (2009). "ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension: A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: Developed in Collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association". Circulation. 119 (16): 2250–2294. doi:10.1161/CIRCULATIONAHA.109.192230. PMID 19332472.
- ^ Barst RJ, McGoon M, Torbicki A; et al. (2004). "Diagnosis and differential assessment of pulmonary arterial hypertension". J. Am. Coll. Cardiol. 43 (12 Suppl S): 40S–47S. doi:10.1016/j.jacc.2004.02.032. PMID 15194177.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sitbon O, Humbert M, Jaïs X; et al. (2005). "Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension". Circulation. 111 (23): 3105–11. doi:10.1161/CIRCULATIONAHA.104.488486. PMID 15939821.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Torres F (2007). "Systematic review of randomised, double-blind clinical trials of oral agents conducted in patients with pulmonary arterial hypertension". Int. J. Clin. Pract. 61 (10): 1756–65. doi:10.1111/j.1742-1241.2007.01545.x. PMID 17877662.
- ^ "UPDATE 1-Encysive gets Canadian approval for hypertension drug". Reuters. 2008-05-30. Retrieved 2007-07-08.
- ^ "U.S. Food and Drug Administration Approves Gilead's Letairis Treatment of Pulmonary Arterial Hypertension" (Press release). Gilead Sciences. 2007-06-15. Retrieved 2007-06-16.
- ^ "FDA approves Adcirca (tadalafil) tablets for pulmonary arterial hypertension" (Press release). 2009-05-26. Retrieved 2010-12-06.
- ^ Forgue ST, Patterson BE, Bedding. AW, et al. Tadalafil pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2005;61:280–288.
- ^ Sally A. Arif, PharmD, BCPS (Department of Pharmacy Practice, Chicago College of Pharmacy, Midwestern University, Downers Grove, Illinois, and Department of Pharmacy, Rush University Medical Center, Chicago, Illinois); and Henry Poon, PharmD, BCPS (Department of Pharmacy, James J. Peters VA Medical Center, Bronx, New York). Tadalafil: A Long-Acting Phosphodiesterase-5 Inhibitor for the Treatment of Pulmonary Arterial Hypertension. 2011;33:993–1004
- ^ Galié N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903.
- ^ "2006 OPTN/SRTR Annual Report". US Scientific Registry of Transplant Recipients. 2006-05-01. Retrieved 2007-03-28.
- ^ McLaughlin VV, Sitbon O, Badesch DB; et al. (2005). "Survival with first-line bosentan in patients with primary pulmonary hypertension". Eur. Respir. J. 25 (2): 244–9. doi:10.1183/09031936.05.00054804. PMID 15684287.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Nauser TD, Stites SW (2001). "Diagnosis and treatment of pulmonary hypertension". Am Fam Physician. 63 (9): 1789–98. PMID 11352291.
- ^ "An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry". Chest. 142 (2): 448–56. August 2012. doi:10.1378/chest.11-1460. PMID 22281797.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) [FREE] - ^ Kelley's essentials of internal medicine. Hagerstwon, MD: Lippincott Williams & Wilkins. 2001. p. 84. ISBN 0-7817-1937-2.
- ^ Edward Benz; David Weatherall; David Warrell; Cox, Timothy J.; Firth, John B. (2005). Oxford textbook of medicine. Oxford [Oxfordshire]: Oxford University Press. p. 1101. ISBN 0-19-856978-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Kaufman, Matthew H.; Latha Stead; Feig, Robert (2007). First aid for the obstetrics & gynecology clerkship. New York: McGraw-Hill, Medical Pub. Division. p. 100. ISBN 0-07-144874-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Ghosh, Amit K. (2008). Mayo Clinic Internal Medicine Review: Eighth Edition (Mayo Clinic Internal Medicine Review). Informa Healthcare. p. 55. ISBN 1-4200-8478-X.
- ^ British Journal of Anaesthesia: "Primary pulmonary hypertension in pregnancy; a role for novel vasodilators" March 19, 2011
- ^ Rudarakanchana, N (November 2001). "New insights into the pathogenesis and treatment of primary pulmonary hypertension". Thorax. 56 (11): 888–890. doi:10.1136/thorax.56.11.888. PMC 1745964. PMID 11641516.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
Sources
- Rubin LJ, Badesch DB (2005). "Evaluation and management of the patient with pulmonary arterial hypertension". Ann. Intern. Med. 143 (4): 282–92. PMID 16103472.
External links
- The Merck Manual Home Edition: Pulmonary Hypertension
- Pulmonary Arterial Hypertension database
- Pulmonary Hypertension Association
- PH Central - the internet resource for Pulmonary Arterial Hypertension
- Facts About Primary Pulmonary Hypertension from the National Heart, Lung, and Blood Institute (NHLBI)
- Webcast: The Changing World of Pulmonary Arterial Hypertension Therapies - American College of CHEST Physicians
- GeneReviews/NCBI/NIH/UW entry on Heritable Pulmonary Arterial Hypertension
- OMIM entries on Heritable Pulmonary Arterial Hypertension
