Loratadine: Difference between revisions
No edit summary |
Undid revision 253138760 by 60.216.166.21 (talk); vandalism |
||
| Line 1: | Line 1: | ||
{{drugbox | |
|||
2-Pyridinemethanol CAS:1121-60-4 Yellow-brown liquid Purity>=98 |
|||
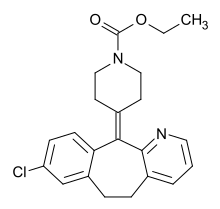
| IUPAC_name = Ethyl 4-(8-chloro-5,6-dihydro-11H-<BR>benzo[5,6]cyclohepta[1,2-b]pyridin-<BR>11-ylidine)-1-piperidinecarboxylate |
|||
3-Pyridinemethanol CAS:500-22-1 Yellow-brown liquid Purity>=98 |
|||
| image = Loratadin.svg |
|||
4-Pyridinemethanol CAS:872-85-5 Light yellow liquid Purity>=98 |
|||
| CAS_number = 79794-75-5 |
|||
| ChemSpiderID = 3820 |
|||
| ATC_prefix = R06 |
|||
| ATC_suffix = AX13 |
|||
| PubChem = 3957 |
|||
| DrugBank = APRD00384 |
|||
| C = 22 | H = 23 | Cl = 1 | N = 2 | O = 2 |
|||
| molecular_weight = 382.88 g/mol |
|||
| smiles = CCOC(=O)N1CCC(=C2c3ccc(Cl)cc3CCc3cccnc23)CC1 |
|||
| bioavailability = N/A due to extensive [[first-pass metabolism]] |
|||
| protein_bound = |
|||
| metabolism = hepatic |
|||
| elimination_half-life = 8 hours (metabolites 12-24 hours) |
|||
| excretion = 40% as conjugated metabolites into urine<br>similar amount into the feces |
|||
| pregnancy_AU = B1 |
|||
| pregnancy_US = B |
|||
| pregnancy_category = |
|||
| legal_UK = GSL |
|||
| legal_US = OTC |
|||
| legal_status = [[Over-the-counter drug|OTC]]<small>([[Canada]])</small> |
|||
| routes_of_administration = oral |
|||
}} |
|||
'''Loratadine''' is a drug used to treat [[allergy|allergies]], and marketed for its non-sedating properties. It is marketed by [[Schering-Plough]] under several trade names such as '''Claritin''', '''Claritin-D''', '''Claritine''', '''Clarityn''', '''Clarityne''' or '''Fristamin''' depending on the market; by Cadila as '''Lorfast'''; by [[Sandoz|Lek]] as '''Lomilan'''; by [[Sandoz]] as '''Symphoral'''; by [[Ranbaxy]] as '''Roletra'''; by [[Pliva]] as '''Rinolan'''; by [[Teva Pharmaceutical Industries|Teva]] as '''AllergyX'''; and by [[Wyeth]] as '''Alavert'''. It is also available as a [[Generic drug|generic]]. In a version marketed as '''Claritin-D''' or '''Clarinase''', loratadine is combined with [[pseudoephedrine]], a decongestant; this makes it somewhat useful for colds as well as allergies, but adds potential side-effects of [[insomnia]], nervousness and anxiety. |
|||
It is considered a second generation agent.<ref name="pmid10444229">{{cite journal |author=Kay GG, Harris AG |title=Loratadine: a non-sedating antihistamine. Review of its effects on cognition, psychomotor performance, mood and sedation |journal=Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology |volume=29 Suppl 3 |issue= |pages=147–50 |year=1999 |month=July |pmid=10444229 |doi= |url=http://www.blackwell-synergy.com/openurl?genre=article&sid=nlm:pubmed&issn=0954-7894&date=1999&volume=29&issue=&spage=147}}</ref> |
|||
2-Picolinic acid methyl ester CAS:2459-07-6 Yellow-brown liquid Purity > = 98 |
|||
3-Picolinic acid methyl ester CAS:93-60-7 Yellow-brown liquid Purity > = 98 |
|||
4-Picolinic acid methyl ester CAS:2459-09-8 Yellow-brown liquid Purity > = 98 |
|||
==Regulation and clinical trials== |
|||
2-Picolinic acid ethyl ester CAS:2524-52-9 Yellow-brown liquid Purity>=98 |
|||
Schering-Plough developed Loratadine as part of a quest for a [[blockbuster drug]], a [[sedative|nonsedating]] [[antihistamine]]. However, by the time Schering submitted the drug to the FDA for approval, the agency had already approved a competitor's nonsedating antihistamine, [[Seldane|Seldane (terfenadine)]], and therefore put Loratadine on a lower priority as a "me too" drug.<ref name="NYTimes">[http://query.nytimes.com/gst/fullpage.html?res=9C07E0D7103BF932A25750C0A9679C8B63&sec=health&spon=&partner=permalink&exprod=permalink The Claritin Effect; Prescription for Profit - New York Times<!-- Bot generated title -->]</ref> Trials also raised questions about whether there was any dose at which Loratadine was simultaneously nonsedating and highly effective. Reviewing a randomized, [[Double blind#Double-blind trials|double-blind trial]], Dr. Sherwin D. Straus of the FDA argued at one point that "10 milligrams is not very different than placebo clinically," and that the reason for making the dose so low was that at higher, more effective doses, it became sedating.<ref name="NYTimes" /> Schering counters that "The innovation of Claritin and the basis for its success are not only that it works, but also that it was the first antihistamine to provide effective relief of allergy symptoms without sedation and with an impeccable safety profile."<ref name="NYTimes" /> |
|||
3-Picolinic acid ethyl ester CAS:614-18-6 Yellow-brown liquid Purity>=99 |
|||
4-Picolinic acid ethyl ester CAS:1570-45-2 Yellow-brown liquid Purity>=99 |
|||
2-Pyridinecarboxylic acid CAS:98-98-6 |
|||
3-Pyridinecarboxylic acid |
|||
Loratadine was eventually approved by the FDA, and in 2001, its last year on patent, it accounted for 28% of Schering's total sales. Although an FDA advisory panel ruled that Loratadine was safe enough to be sold over the counter, Schering opposed such a decision on the grounds that it would reduce the price that could be charged for the drug.<ref>[http://www.therubins.com/legal/patext.htm Patents and Prescription Drugs<!-- Bot generated title -->]</ref> The drug continued to be available only by prescription in the U.S. until it went off patent in 2002. It was then immediately approved for over-the-counter sales. Once it became an unpatented over-the-counter drug, the price dropped precipitously, and insurance companies no longer paid for it. In response, Schering launched an expensive advertising campaign to convince users to switch to [[Clarinex]] (Desloratadine), which is a metabolized form of Loratadine. A 2003 study comparing the two drugs found that "There is no clinical advantage to switching a patient from loratadine to desloratadine. However, it may be an option for patients whose medical insurance no longer covers loratadine if the co-pay is less than the cost of the over-the-counter product."<ref>{{cite journal | author=See S | title=Desloratadine for allergic rhinitis | journal=Am Fam Physician | volume=68 | issue=10 | pages=2015–6 | year=2003 | pmid=14655812 | url=http://www.aafp.org/afp/20031115/steps.html}}</ref> |
|||
In the [[United States|U.S.]] and [[United Kingdom|UK]], loratadine is the only drug of its class available [[Over-the-counter drug|over the counter]] (though it is no longer the only second generation antihistamine available in this manner). In the UK, larger quantities are only available over the counter; they are "P-Line" and can only be sold in the presence of a [[pharmacist]]. However, packets of up to and including 7 tablets are available "off the shelf" (GSL). [[Desloratadine]] is an over the counter drug in [[Canada]], but is a prescription drug in the U.S.. |
|||
contact:Alice |
|||
phone:+86-531-82375818 |
|||
{{further|[[desloratadine]]}} |
|||
Tel:013969142597 |
|||
MSN:xuezhongyan888@hotmail.com |
|||
==Drug profile== |
|||
[[Image:Loratadine10mg.png|thumb|Loratadine 10 mg (Rx)]] |
|||
====Forms==== |
|||
Loratadine is available as tablets, oral suspension and syrup, and also in combination with [[pseudoephedrine]]. |
|||
Also available are quick-dissolving tablets, which are marketed as being faster to get into one's circulatory system but which require special handling to avoid degrading in the package. |
|||
====Indications==== |
|||
Loratadine is indicated for the [[symptoms|symptomatic]] relief of allergy such as [[hay fever]] (allergic rhinitis), [[urticaria]] (hives), and other skin allergies. |
|||
For allergic rhinitis (hay fever), loratadine is effective for both nasal and eye symptoms: sneezing, runny nose, itchy or burning eyes. |
|||
====Mechanism of action==== |
|||
Loratadine is a tricyclic [[antihistamine]], which selectively antagonizes peripheral [[histamine]] [[histamine H1 receptor|H<sub>1</sub>-receptors]]. Histamine is responsible for many features of allergic reactions. |
|||
Loratadine has a long-lasting effect and does not normally cause [[drowsiness]] because it does not readily enter the [[central nervous system]] (see ''Side-effects'' section below). |
|||
====Pharmacokinetics==== |
|||
Loratadine is given orally, is well absorbed from the [[gastrointestinal tract]], and has rapid [[first-pass metabolism|first-pass hepatic metabolism]]. Loratadine is almost totally bound to [[plasma proteins]]. Its metabolite, desloratadine (descarboethoxyloratadine), is also active, but binds to plasma proteins only moderately. |
|||
Loratadine's peak effect occurs in 1-2 hours, and its biological [[half-life]] is on average 8 hours with its metabolite's half-life being 28 hours. About 40% is excreted as conjugated metabolites into the urine, and a similar amount is excreted into the feces. Traces of unmetabolised loratadine can be found in the urine. |
|||
====Side-effects==== |
|||
;Non-sedating antihistamine |
|||
As a non-sedating [[antihistamine]], loratadine causes less [[sedation]] and [[psychomotor]] [[impairment]] than the older antihistamines because it penetrates the [[blood brain barrier]] only to a slight extent. |
|||
Although drowsiness is rare, patients should nevertheless be advised that it can occur and may affect performance of skilled tasks (e.g. driving); excess [[alcohol]] should be avoided. |
|||
;Most common side-effects |
|||
[[Drowsiness]], headache, psychomotor impairment, and antimuscarinic effects such as urinary retention, dry mouth, blurred vision, and gastrointestinal disturbances are the most common side effects. |
|||
;Other rarer side-effects |
|||
[[Hypotension]], [[extrapyramidal effects]], dizziness, confusion, depression, sleep disturbances, lower back pain, tremor, convulsions, palpitation, arrhythmias, hypersensitivity reactions (including bronchospasm, angioedema, and anaphylaxis, rashes, and photosensitivity reactions), blood disorders, liver dysfunction, and angle-closure glaucoma are less common side effects. |
|||
====Cautions and contradications==== |
|||
Loratadine should be used with caution in [[hepatic]] disease and dose reduction may be necessary in [[renal]] impairment. Caution may be required in [[epilepsy]]. Children and the elderly are more susceptible to side-effects (see ''Side-effects'' section above). Loratadine is a category L-2 (classified by the [[American Academy of Pediatrics]] as a drug "Usually Compatible With [[Breast-feeding]]"<ref>{{cite journal | title=Transfer of drugs and other chemicals into human milk | journal=Pediatrics | volume=108 | issue=3 | pages=776–89 | year=2001 | url=http://aappolicy.aappublications.org/cgi/content/full/pediatrics;108/3/776 | pmid=11533352 }}</ref>) and category B in [[pregnancy]]<ref>[http://www.aafp.org/afp/20031115/steps.html STEPS - November 15, 2003 - American Family Physician<!-- Bot generated title -->]</ref>. |
|||
==References== |
|||
{{reflist|2}} |
|||
==External links== |
|||
* [http://www.nlm.nih.gov/medlineplus/druginfo/medmaster/a697038.html Loratadine] — MedlinePlus Drug Information, U.S. National Library of Medicine, National Institutes of Health |
|||
* [http://www.rxlist.com/cgi/generic/lorat.htm Claritin (loratadine) drug description] — RxList (Internet Drug Index) |
|||
* [http://www.claritin.com/images/for_me/products/tablets/tablets_back_pop.pdf Claritin] — patient information leaflet |
|||
* [http://www.claratyne.com.au Claratyne] - For Australia |
|||
* [http://redpoll.pharmacy.ualberta.ca/drugbank/cgi-bin/getCard.cgi?CARD=APRD00384.txt DrugBank: Loratadine] — Wishart DS et al., ''DrugBank: a comprehensive resource for in silico drug discovery and exploration''. ''Nucleic Acids Res''. ''2006 1;34'' |
|||
* [http://query.nytimes.com/gst/fullpage.html?res=9C07E0D7103BF932A25750C0A9679C8B63&sec=health&spon=&partner=permalink&exprod=permalink The Claritin Effect; Prescription for Profit] - a New York Times Magazine article about the marketing of Claritin as a blockbuster drug |
|||
==See also== |
|||
{{antihistamines}} |
|||
[[Category:H1 receptor antagonists]] |
|||
[[Category:Carbamates]] |
|||
[[Category:Schering-Plough]] |
|||
[[de:Loratadin]] |
|||
[[es:Loratadina]] |
|||
[[fr:Loratadine]] |
|||
[[hu:Loratadin]] |
|||
[[nl:Loratadine]] |
|||
[[ja:ロラタジン]] |
|||
[[pl:Loratadyna]] |
|||
[[pt:Loratadina]] |
|||
[[ru:Лоратадин]] |
|||
[[sr:Лоратидин]] |
|||
[[sv:Loratadin]] |
|||
[[th:ลอราทาดีน]] |
|||
[[uk:Лоратадин]] |
|||
Revision as of 06:35, 21 November 2008
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A due to extensive first-pass metabolism |
| Metabolism | hepatic |
| Elimination half-life | 8 hours (metabolites 12-24 hours) |
| Excretion | 40% as conjugated metabolites into urine similar amount into the feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.120.122 |
| Chemical and physical data | |
| Formula | C22H23ClN2O2 |
| Molar mass | 382.88 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
Loratadine is a drug used to treat allergies, and marketed for its non-sedating properties. It is marketed by Schering-Plough under several trade names such as Claritin, Claritin-D, Claritine, Clarityn, Clarityne or Fristamin depending on the market; by Cadila as Lorfast; by Lek as Lomilan; by Sandoz as Symphoral; by Ranbaxy as Roletra; by Pliva as Rinolan; by Teva as AllergyX; and by Wyeth as Alavert. It is also available as a generic. In a version marketed as Claritin-D or Clarinase, loratadine is combined with pseudoephedrine, a decongestant; this makes it somewhat useful for colds as well as allergies, but adds potential side-effects of insomnia, nervousness and anxiety.
It is considered a second generation agent.[2]
Regulation and clinical trials
Schering-Plough developed Loratadine as part of a quest for a blockbuster drug, a nonsedating antihistamine. However, by the time Schering submitted the drug to the FDA for approval, the agency had already approved a competitor's nonsedating antihistamine, Seldane (terfenadine), and therefore put Loratadine on a lower priority as a "me too" drug.[3] Trials also raised questions about whether there was any dose at which Loratadine was simultaneously nonsedating and highly effective. Reviewing a randomized, double-blind trial, Dr. Sherwin D. Straus of the FDA argued at one point that "10 milligrams is not very different than placebo clinically," and that the reason for making the dose so low was that at higher, more effective doses, it became sedating.[3] Schering counters that "The innovation of Claritin and the basis for its success are not only that it works, but also that it was the first antihistamine to provide effective relief of allergy symptoms without sedation and with an impeccable safety profile."[3]
Loratadine was eventually approved by the FDA, and in 2001, its last year on patent, it accounted for 28% of Schering's total sales. Although an FDA advisory panel ruled that Loratadine was safe enough to be sold over the counter, Schering opposed such a decision on the grounds that it would reduce the price that could be charged for the drug.[4] The drug continued to be available only by prescription in the U.S. until it went off patent in 2002. It was then immediately approved for over-the-counter sales. Once it became an unpatented over-the-counter drug, the price dropped precipitously, and insurance companies no longer paid for it. In response, Schering launched an expensive advertising campaign to convince users to switch to Clarinex (Desloratadine), which is a metabolized form of Loratadine. A 2003 study comparing the two drugs found that "There is no clinical advantage to switching a patient from loratadine to desloratadine. However, it may be an option for patients whose medical insurance no longer covers loratadine if the co-pay is less than the cost of the over-the-counter product."[5]
In the U.S. and UK, loratadine is the only drug of its class available over the counter (though it is no longer the only second generation antihistamine available in this manner). In the UK, larger quantities are only available over the counter; they are "P-Line" and can only be sold in the presence of a pharmacist. However, packets of up to and including 7 tablets are available "off the shelf" (GSL). Desloratadine is an over the counter drug in Canada, but is a prescription drug in the U.S..
Drug profile

Forms
Loratadine is available as tablets, oral suspension and syrup, and also in combination with pseudoephedrine.
Also available are quick-dissolving tablets, which are marketed as being faster to get into one's circulatory system but which require special handling to avoid degrading in the package.
Indications
Loratadine is indicated for the symptomatic relief of allergy such as hay fever (allergic rhinitis), urticaria (hives), and other skin allergies.
For allergic rhinitis (hay fever), loratadine is effective for both nasal and eye symptoms: sneezing, runny nose, itchy or burning eyes.
Mechanism of action
Loratadine is a tricyclic antihistamine, which selectively antagonizes peripheral histamine H1-receptors. Histamine is responsible for many features of allergic reactions.
Loratadine has a long-lasting effect and does not normally cause drowsiness because it does not readily enter the central nervous system (see Side-effects section below).
Pharmacokinetics
Loratadine is given orally, is well absorbed from the gastrointestinal tract, and has rapid first-pass hepatic metabolism. Loratadine is almost totally bound to plasma proteins. Its metabolite, desloratadine (descarboethoxyloratadine), is also active, but binds to plasma proteins only moderately.
Loratadine's peak effect occurs in 1-2 hours, and its biological half-life is on average 8 hours with its metabolite's half-life being 28 hours. About 40% is excreted as conjugated metabolites into the urine, and a similar amount is excreted into the feces. Traces of unmetabolised loratadine can be found in the urine.
Side-effects
- Non-sedating antihistamine
As a non-sedating antihistamine, loratadine causes less sedation and psychomotor impairment than the older antihistamines because it penetrates the blood brain barrier only to a slight extent.
Although drowsiness is rare, patients should nevertheless be advised that it can occur and may affect performance of skilled tasks (e.g. driving); excess alcohol should be avoided.
- Most common side-effects
Drowsiness, headache, psychomotor impairment, and antimuscarinic effects such as urinary retention, dry mouth, blurred vision, and gastrointestinal disturbances are the most common side effects.
- Other rarer side-effects
Hypotension, extrapyramidal effects, dizziness, confusion, depression, sleep disturbances, lower back pain, tremor, convulsions, palpitation, arrhythmias, hypersensitivity reactions (including bronchospasm, angioedema, and anaphylaxis, rashes, and photosensitivity reactions), blood disorders, liver dysfunction, and angle-closure glaucoma are less common side effects.
Cautions and contradications
Loratadine should be used with caution in hepatic disease and dose reduction may be necessary in renal impairment. Caution may be required in epilepsy. Children and the elderly are more susceptible to side-effects (see Side-effects section above). Loratadine is a category L-2 (classified by the American Academy of Pediatrics as a drug "Usually Compatible With Breast-feeding"[6]) and category B in pregnancy[7].
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Kay GG, Harris AG (1999). "Loratadine: a non-sedating antihistamine. Review of its effects on cognition, psychomotor performance, mood and sedation". Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 29 Suppl 3: 147–50. PMID 10444229.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c The Claritin Effect; Prescription for Profit - New York Times
- ^ Patents and Prescription Drugs
- ^ See S (2003). "Desloratadine for allergic rhinitis". Am Fam Physician. 68 (10): 2015–6. PMID 14655812.
- ^ "Transfer of drugs and other chemicals into human milk". Pediatrics. 108 (3): 776–89. 2001. PMID 11533352.
- ^ STEPS - November 15, 2003 - American Family Physician
External links
- Loratadine — MedlinePlus Drug Information, U.S. National Library of Medicine, National Institutes of Health
- Claritin (loratadine) drug description — RxList (Internet Drug Index)
- Claritin — patient information leaflet
- Claratyne - For Australia
- DrugBank: Loratadine — Wishart DS et al., DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006 1;34
- The Claritin Effect; Prescription for Profit - a New York Times Magazine article about the marketing of Claritin as a blockbuster drug
