Chemistry of ascorbic acid: Difference between revisions
Undid revision 413975042 by 99.249.34.65 (talk) vandalism |
No edit summary |
||
| Line 57: | Line 57: | ||
}} |
}} |
||
}} |
}} |
||
'''Ascorbic acid''' is a [[sugar acid]] with [[antioxidant]] properties. Its appearance is white to light-yellow crystals or powder, and it is water-soluble. One form of ascorbic acid is commonly known as [[vitamin C]]. The name is derived from ''a-'' (meaning "no") and ''scorbutus'' ([[scurvy]]), the disease caused by a deficiency of vitamin C. In 1937 the [[Nobel Prize]] for chemistry was awarded to [[ |
'''Ascorbic acid''' is a [[sugar acid]] with [[antioxidant]] properties. Its appearance is white to light-yellow crystals or powder, and it is water-soluble. One form of ascorbic acid is commonly known as [[vitamin C]]. The name is derived from ''a-'' (meaning "no") and ''scorbutus'' ([[scurvy]]), the disease caused by a deficiency of vitamin C. |
||
In 1937 the [[Nobel Prize]] for chemistry was awarded to [[Norman Haworth]] for his work in determining the structure of ascorbic acid (shared with [[Paul Karrer]], who received his award for work on [[vitamin]]s), and the prize for Physiology or Medicine that year went to [[Albert Szent-Györgyi]] for his studies of the biological functions of L-ascorbic acid. At the time of its discovery in the 1920s, it was called '''hexuronic acid''' by some researchers, but named L-ascorbic acid by Haworth and Szent-Györgyi when its structure was finally proven by synthesis.<ref>{{citation | first1 = Joseph Louis | last1 = Svirbelf | first2 = Albert | last2 = Szent-Györgyi | authorlink2 = Albert Szent-Györgyi | url = http://profiles.nlm.nih.gov/WG/B/B/G/W/_/wgbbgw.pdf | title = The Chemical Nature Of Vitamin C | date = April 25, 1932}}. Part of the [[National Library of Medicine]] collection. Accessed January 2007</ref> It is an enzyme cofactor in [[tyrosine]] [[oxidation]].<ref>http://www.jbc.org/content/196/2/761.full.pdf</ref> It creates volatile compounds when mixed with [[glucose]] and [[amino acids]].<ref>http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1981.tb15349.x/abstract</ref> |
|||
==History== |
|||
The discovery of a essential disease preventing compound in foods distinct from the one that prevented beriberi, was made in 1907 by two Norwegian physicians investigating dietary deficiency diseases using a new model of [[ginnea pig]]s. Guinea pigs proved to be succeptable to scurvy, and the factor foods they needed was eventually called [[vitamin C]]. |
|||
From 1928 to 1932, the Hungarian research team led by [[Albert Szent-Györgyi]] and that of the [[United States|American]] worker [[Charles Glen King]], identified the anti-scorbutic factor. Szent-Györgyi had isolated the chemical hexuronic acid from animal adrenal glands at the Mayo clinic, and suspected it to be the antiscorbutic factor, but could not prove it without a biological assay. This was finally done by King's laboratory at the University of Pittsburgh. In late 1931, King's lab obtained adrenal hexuronic acid indirectly from Szent-Györgyi and proven that it was vitamin C by 1932. Later that year, Szent-Györgyi's group discovered that paprika peppers, a common spice in the Hungarian diet, was a rich source of hexuronic acid, and he sent some of the now far more available chemical to [[Norman Haworth]], a noted British sugar chemist.<ref>[http://profiles.nlm.nih.gov/WG/Views/Exhibit/narrative/szeged.html Story of Vitamin C's chemical discovery. Accessed Jan 21, 2010]</ref> |
|||
In 1933, working with the then Assistant Director of Research (later Sir) [[Edmund Hirst]] and their research teams, Haworth deduced the correct structure and optical-isomeric nature of vitamin C, and reported the first synthesisis of the vitamin.<ref>{{Citation |
|||
| last = Davies |
|||
| first = Michael B. |
|||
| last2 = Austin |
|||
| first2 = John |
|||
| last3 = Partridge |
|||
| first3 = David A. |
|||
| title = Vitamin C: Its Chemistry and Biochemistry |
|||
| publisher = The Royal Society of Chemistry |
|||
| year = 1991 |
|||
| page = 48 |
|||
| isbn = 0-85186-333-7}} |
|||
</ref> In honor of the compound's antiscorbutic properties, Haworth and Szent-Györgyi now proposed the new name of "a-scorbic acid" for the molecule, with [[L-ascorbic acid]] as its formal chemical name. |
|||
== Chemistry == |
== Chemistry == |
||
Revision as of 18:53, 28 February 2011
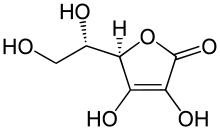
| |

| |
| Names | |
|---|---|
| IUPAC name
(5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one
| |
| Other names
Vitamin C
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| E number | E300 (antioxidants, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.124 g·mol−1 |
| Appearance | White or light yellow solid |
| Density | 1.65 g/cm3 |
| 33 g/100 mL | |
| Solubility in ethanol | 2 g/100 mL |
| Solubility in glycerol | 1 g/100 mL |
| Solubility in propylene glycol | 5 g/100 mL |
| Solubility in other solvents | insoluble in diethyl ether, chloroform, benzene, petroleum ether, oils, fats |
| Acidity (pKa) | 4.10 (first), 11.6 (second) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
11.9 g/kg (oral, rat)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ascorbic acid is a sugar acid with antioxidant properties. Its appearance is white to light-yellow crystals or powder, and it is water-soluble. One form of ascorbic acid is commonly known as vitamin C. The name is derived from a- (meaning "no") and scorbutus (scurvy), the disease caused by a deficiency of vitamin C.
In 1937 the Nobel Prize for chemistry was awarded to Norman Haworth for his work in determining the structure of ascorbic acid (shared with Paul Karrer, who received his award for work on vitamins), and the prize for Physiology or Medicine that year went to Albert Szent-Györgyi for his studies of the biological functions of L-ascorbic acid. At the time of its discovery in the 1920s, it was called hexuronic acid by some researchers, but named L-ascorbic acid by Haworth and Szent-Györgyi when its structure was finally proven by synthesis.[2] It is an enzyme cofactor in tyrosine oxidation.[3] It creates volatile compounds when mixed with glucose and amino acids.[4]
History
The discovery of a essential disease preventing compound in foods distinct from the one that prevented beriberi, was made in 1907 by two Norwegian physicians investigating dietary deficiency diseases using a new model of ginnea pigs. Guinea pigs proved to be succeptable to scurvy, and the factor foods they needed was eventually called vitamin C.
From 1928 to 1932, the Hungarian research team led by Albert Szent-Györgyi and that of the American worker Charles Glen King, identified the anti-scorbutic factor. Szent-Györgyi had isolated the chemical hexuronic acid from animal adrenal glands at the Mayo clinic, and suspected it to be the antiscorbutic factor, but could not prove it without a biological assay. This was finally done by King's laboratory at the University of Pittsburgh. In late 1931, King's lab obtained adrenal hexuronic acid indirectly from Szent-Györgyi and proven that it was vitamin C by 1932. Later that year, Szent-Györgyi's group discovered that paprika peppers, a common spice in the Hungarian diet, was a rich source of hexuronic acid, and he sent some of the now far more available chemical to Norman Haworth, a noted British sugar chemist.[5]
In 1933, working with the then Assistant Director of Research (later Sir) Edmund Hirst and their research teams, Haworth deduced the correct structure and optical-isomeric nature of vitamin C, and reported the first synthesisis of the vitamin.[6] In honor of the compound's antiscorbutic properties, Haworth and Szent-Györgyi now proposed the new name of "a-scorbic acid" for the molecule, with L-ascorbic acid as its formal chemical name.
Chemistry
Acidity
Ascorbic acid behaves as a vinylogous carboxylic acid where the electrons in the double bond, hydroxyl group lone pair, and the carbonyl double bond form a conjugated system. Because the two major resonance structures stabilize the deprotonated conjugate base of ascorbic acid, the hydroxyl group in ascorbic acid is much more acidic than typical hydroxyl groups. In other words, ascorbic acid can be considered an enol where the deprotonated form is a stabilized enolate.
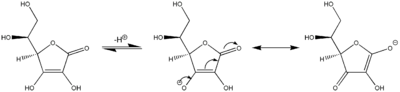
Tautomerism
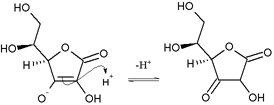
Ascorbic acid also interconverts into two unstable ketone tautomers by proton transfer, although it is the most stable in the enol form. The proton of the hydroxyl of the enol is removed. Then a pair of electrons from the resulting oxide anion pushes down to form the ketone at the 2 or 3 position and the electrons from the double bond move to the 3 or 2 position, respectively, forming the carbanion, which picks up the proton resulting in two possible forms: 1-carboxyl-2-ketone and 1-carboxyl-3-ketone.
Determination
The concentration of a solution of ascorbic acid can be determined in many ways, the most common ways involving titration with an oxidizing agent.
- Iodine
Another method involves using iodine and a starch indicator, wherein iodine reacts with ascorbic acid, and, when all the ascorbic acid has reacted, the iodine is then in excess, forming a blue-black complex with the starch indicator. This indicates the end-point of the titration. As an alternative, ascorbic acid can be treated with iodine in excess, followed by back titration with sodium thiosulfate using starch as an indicator.[7]
- Iodate and iodine
The above \ involving iodine requires making up and standardising the iodine solution. One way around this is to generate the iodine in the presence of the ascorbic acid by the reaction of iodate and iodide ion in acid solution, the ionic equation for this reaction follows;
- IO3− + 5 I−
+ 6 H+
→ 3 I
2 + 3 H
2O
- N-Bromosuccinimide
A much-less-common oxidising agent is N-bromosuccinimide, (NBS). In this titration, the NBS oxidises the ascorbic acid (in the presence of potassium iodide and starch). When the NBS is in excess (i.e., the reaction is complete), the NBS liberates the iodine from the potassium iodide, which then forms the blue/black complex with starch, indicating the end-point of the titration.
The product of Ascorbic acids chemical makeup is 40% Vitamin C.
- Iodimetric Determination involving electrochemical method
Electrolyze the solution of potassium iodide to produce iodine and react with ascorbic acid spontaneously, the end of process is determined by the current rise method. The amount of ascorbic acid can be calculated by the Faraday's law.
Antioxidant mechanism
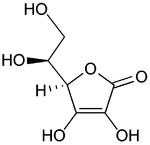
|
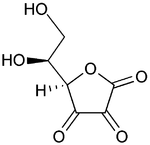
|
(reduced form of Vitamin C)
Bottom: dehydroascorbic acid
(nominal oxidized form of Vitamin C)
Ascorbate usually acts as an antioxidant by being available for energetically favourable oxidation. Many oxidants (typically, reactive oxygen species) such as the hydroxyl radical (formed from hydrogen peroxide), contain an unpaired electron, and, thus, are highly reactive. This can be highly damaging to humans and plants at the molecular level due to their possible interaction with nucleic acids, proteins, and lipids. These free radical interactions are damaging since they result in a whole chain of free radical reactions. More specifically, the interaction of an initial free radical (often reactive oxygen species) with another molecule changes that molecule itself into a free radical, which then reacts with other molecules, also turning them into free radicals. Ascorbate can terminate these chained radical reactions by serving as a stable (electron + proton) donor in interactions with free radicals, being converted into the
radical ion called "semidehydroascorbate" and then dehydroascorbate. The net reaction is RO• + C
6H
7O−
6 → ROH + C
6H
6O-•
6. The oxidized forms of ascorbate are relatively unreactive, and do not cause cellular damage. They can be converted back to ascorbate by cellular enzymes.
However, being a good electron donor, excess ascorbate in the presence of free metal ions can not only promote, but also initiate free radical reactions, thus making it a potentially dangerous pro-oxidative compound in certain metabolic contexts.
Uses
Ascorbic acid is easily oxidized and so is used as a reductant in photographic developer solutions (among others) and as a preservative.
Exposure to oxygen, metals, light, or heat destroys ascorbic acid, so it must be stored in a dark, cold, and non-metallic container.
The L-enantiomer of ascorbic acid is also known as vitamin C. The name "ascorbic" comes from its property of preventing and curing scurvy. Primates, including humans, and a few other species in all divisions of the animal kingdom, notably the guinea pig, have lost the ability to synthesize ascorbic acid, and must obtain it in their food.
Ascorbic acid and its sodium, potassium, and calcium salts are commonly used as antioxidant food additives. These compounds are water-soluble and thus cannot protect fats from oxidation: For this purpose, the fat-soluble esters of ascorbic acid with long-chain fatty acids (ascorbyl palmitate or ascorbyl stearate) can be used as food antioxidants. Eighty percent of the world's supply of ascorbic acid is produced in China.[8]
The relevant European food additive E numbers are
- E300 ascorbic acid,
- E301 sodium ascorbate,
- E302 calcium ascorbate,
- E303 potassium ascorbate,
- E304 fatty acid esters of ascorbic acid (i) ascorbyl palmitate (ii) ascorbyl stearate.
In plastic manufacturing, ascorbic acid can be used to assemble molecular chains more quickly and with less waste than traditional synthesis methods.[9]
Ascorbic acid synthesis in living organisms
Ascorbic acid is found in plants, animals, and single-cell organisms.[10] All animals either make it, eat it, or else die from scurvy due to lack of it. Reptiles and older orders of birds make ascorbic acid in their kidneys. Recent orders of birds and most mammals make ascorbic acid in their liver where the enzyme L-gulonolactone oxidase is required to convert glucose to ascorbic acid.[10] Humans, some other primates, and guinea pigs are not able to make L-gulonolactone oxidase because of a genetic mutation and are therefore unable to make ascorbic acid. Synthesis and signalling properties are still under investigation.[11]
Industrial preparation
Ascorbic acid is synthesized from glucose through a five-step process. Firstly, glucose, a pentahydroxy aldose, is reduced to sorbitol, which is then oxidized by the microorganism Acetobacter suboxydans. To selectively oxidize only one of the six hydroxy groups in sorbitol, an enzymatic reaction is involved. Treatment with propanone (acetone) and an acid catalyst then protects four of the remaining hydroxyl groups in acetal linkages. The unprotected hydroxyl group is chemically oxidized to the carboxylic acid by reaction with sodium hypochlorite (bleaching solution). Hydrolysis with acid then removes the two acetal groups. The removal then causes an internal ester-forming reaction to yield ascorbic acid. Each of the five steps has a yield larger than 90%. The industrial synthesis of ascorbic acid from glucose
Compendial status
See also
- Colour retention agent
- Vitamin C: a discussion of the medical properties of ascorbic acid as well as its historic and social role
- Dehydroascorbic acid, an oxidized form of ascorbic acid.
- Erythorbic acid: a diastereomer of ascorbic acid.
- Mineral ascorbates: salts of ascorbic acid
- D-erythroascorbic acid: yeasts do not make vitamin C (L-ascorbic acid), but a similar antioxidant known as D-erythroascorbic acid
- Acids in wine
Notes and references
- ^ "Safety (MSDS) data for ascorbic acid". Oxford University. 2005-10-09. Retrieved 2007-02-21.
- ^ Svirbelf, Joseph Louis; Szent-Györgyi, Albert (April 25, 1932), The Chemical Nature Of Vitamin C (PDF). Part of the National Library of Medicine collection. Accessed January 2007
- ^ http://www.jbc.org/content/196/2/761.full.pdf
- ^ http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.1981.tb15349.x/abstract
- ^ Story of Vitamin C's chemical discovery. Accessed Jan 21, 2010
- ^ Davies, Michael B.; Austin, John; Partridge, David A. (1991), Vitamin C: Its Chemistry and Biochemistry, The Royal Society of Chemistry, p. 48, ISBN 0-85186-333-7
- ^ A website with an excerpt to using iodine
- ^ Weiss, Rick (May 20, 2007), "Tainted Chinese Imports Common", Washington Post, retrieved 2010-04-25.
- ^ Vitamin C, water have benefits for plastic manufacturing, Reliable Plant Magazine, 2007, archived from the original on 2007-09-27, retrieved 2007-06-25.
- ^ a b Stone, Irwin (1972), The Natural History of Ascorbic Acid in the Evolution of Mammals and Primates.
- ^ Valpuesta, Victoriano; Botella, Miguel (2004), "Biosynthesis of L-ascorbic acid in plants: new pathways for an old antioxidant" (PDF), TRENDS in Plant Science, 9 (12)
{{citation}}: Unknown parameter|month=ignored (help) - ^ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Retrieved 4 February 2010.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Japanese Pharmacopoeia, Fifteenth Edition" (PDF). 2006. Retrieved 4 Februally 2010.
{{cite web}}: Check date values in:|accessdate=(help); Cite has empty unknown parameter:|coauthors=(help)
Further reading
- Clayden; Greeves; Warren; Wothers (2001), Organic Chemistry, Oxford University Press, ISBN 0-19-850346-6.
- Davies, Michael B.; Austin, John; Partridge, David A., Vitamin C: Its Chemistry and Biochemistry, Royal Society of Chemistry, ISBN 0-85186-333-7.
- Coultate, T. P., Food: The Chemistry of Its Components (3rd ed.), Royal Society of Chemistry, ISBN 0-85404-513-9.
- Gruenwald, J.; Brendler, T.; Jaenicke, C., eds. (2004), PDR for Herbal Medicines (3rd ed.), Montvale, New Jersey: Thomson PDR.
- McMurry, John (2008), Organic Chemistry (7e ed.), Thomson Learning, ISBN 978-0-495-11628-8.
External links
- International Chemical Safety Card 0379
- SIDS Initial Assessment Report for L-Ascorbic acid from the Organisation for Economic Co-operation and Development (OECD)
- IPCS Poisons Information Monograph (PIM) 046
- Interactive 3D-structure of vitamin C with details on the x-ray structure
