Bubonic plague
| Bubonic plague | |
|---|---|
 | |
| A bubo on the upper thigh of a person infected with bubonic plague | |
| Specialty | Infectious disease |
| Symptoms | Fever, headaches, vomiting, swollen lymph nodes[1][2] |
| Complications | Gangrene, meningitis[3] |
| Usual onset | 1–7 days after exposure[1] |
| Causes | Yersinia pestis spread by fleas[1] |
| Diagnostic method | Finding the bacterium in the blood, sputum, or lymph nodes[1] |
| Treatment | Antibiotics such as streptomycin, gentamicin, or doxycycline[4][5] |
| Frequency | 650 cases reported a year[1] |
| Deaths | 10% mortality with treatment[4] 30–90% if untreated[1][4] |
Bubonic plague is one of three types of plague caused by the bacterium Yersinia pestis.[1] One to seven days after exposure to the bacteria, flu-like symptoms develop.[1] These symptoms include fever, headaches, and vomiting,[1] as well as swollen and painful lymph nodes occurring in the area closest to where the bacteria entered the skin.[2] Acral necrosis, the dark discoloration of skin, is another symptom. Occasionally, swollen lymph nodes, known as "buboes", may break open.[1]
The three types of plague are the result of the route of infection: bubonic plague, septicemic plague, and pneumonic plague.[1] Bubonic plague is mainly spread by infected fleas from small animals.[1] It may also result from exposure to the body fluids from a dead plague-infected animal.[6] Mammals such as rabbits, hares, and some cat species are susceptible to bubonic plague, and typically die upon contraction.[7] In the bubonic form of plague, the bacteria enter through the skin through a flea bite and travel via the lymphatic vessels to a lymph node, causing it to swell.[1] Diagnosis is made by finding the bacteria in the blood, sputum, or fluid from lymph nodes.[1]
Prevention is through public health measures such as not handling dead animals in areas where plague is common.[8][1] While vaccines against the plague have been developed, the World Health Organization recommends that only high-risk groups, such as certain laboratory personnel and health care workers, get inoculated.[1] Several antibiotics are effective for treatment, including streptomycin, gentamicin, and doxycycline.[4][5]
Without treatment, plague results in the death of 30% to 90% of those infected.[1][4] Death, if it occurs, is typically within 10 days.[9] With treatment, the risk of death is around 10%.[4] Globally between 2010 and 2015 there were 3,248 documented cases, which resulted in 584 deaths.[1] The countries with the greatest number of cases are the Democratic Republic of the Congo, Madagascar, and Peru.[1]
The plague is considered the likely cause of the Black Death that swept through Asia, Europe, and Africa in the 14th century and killed an estimated 50 million people,[1][10] including about 25% to 60% of the European population.[1][11] Because the plague killed so many of the working population, wages rose due to the demand for labor.[11] Some historians see this as a turning point in European economic development.[11] The disease is also considered to have been responsible for the Plague of Justinian, originating in the Eastern Roman Empire in the 6th century CE, as well as the third epidemic, affecting China, Mongolia, and India, originating in the Yunnan Province in 1855.[12] The term bubonic is derived from the Greek word βουβών, meaning "groin."[13]
Cause

The bubonic plague is an infection of the lymphatic system, usually resulting from the bite of an infected flea, Xenopsylla cheopis (the Oriental rat flea).[14] Several flea species carried the bubonic plague, such as Pulex irritans (the human flea), Xenopsylla cheopis, and Ceratophyllus fasciatus.[14] Xenopsylla cheopis was the most effective flea species for transmission.[14]
The flea is parasitic on house and field rats and seeks out other prey when its rodent host dies. Rats were an amplifying factor to bubonic plague due to their common association with humans as well as the nature of their blood.[15] The rat's blood allows the rat to withstand a major concentration of the plague.[15] The bacteria form aggregates in the gut of infected fleas, and this results in the flea regurgitating ingested blood, which is now infected, into the bite site of a rodent or human host. Once established, the bacteria rapidly spread to the lymph nodes of the host and multiply. The fleas that transmit the disease only directly infect humans when the rat population in the area is wiped out from a mass infection.[16] Furthermore, in areas with a large population of rats, the animals can harbor low levels of the plague infection without causing human outbreaks.[15] With no new rat inputs being added to the population from other areas, the infection only spread to humans in very rare cases of overcrowding.[15]
Signs and symptoms
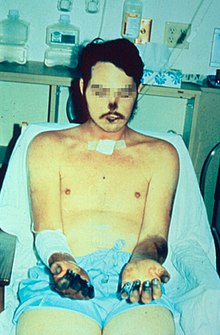
After being transmitted via the bite of an infected flea, the Y. pestis bacteria become localized in an inflamed lymph node, where they begin to colonize and reproduce. Infected lymph nodes develop hemorrhages, which result in the death of tissue.[17] Y. pestis bacilli can resist phagocytosis and even reproduce inside phagocytes and kill them. As the disease progresses, the lymph nodes can hemorrhage and become swollen and necrotic. Bubonic plague can progress to lethal septicemic plague in some cases. The plague is also known to spread to the lungs and become the disease known as the pneumonic plague. Symptoms appear 2–7 days after getting bitten and they include:[14]
- Chills
- General ill feeling (malaise)
- High fever >39 °C (102.2 °F)
- Muscle cramps[17]
- Seizures
- Smooth, painful lymph gland swelling called a bubo, commonly found in the groin, but may occur in the armpits or neck, most often near the site of the initial infection (bite or scratch)
- Pain may occur in the area before the swelling appears
- Gangrene of the extremities such as toes, fingers, lips, and tip of the nose.[18]
The best-known symptom of bubonic plague is one or more infected, enlarged, and painful lymph nodes, known as buboes. Buboes associated with the bubonic plague are commonly found in the armpits, upper femoral area, groin, and neck region. These buboes will grow and become more painful over time, often to the point of bursting.[19] Symptoms include heavy breathing, continuous vomiting of blood (hematemesis), aching limbs, coughing, and extreme pain caused by the decay or decomposition of the skin while the person is still alive. Additional symptoms include extreme fatigue, gastrointestinal problems, spleen inflammation, lenticulae (black dots scattered throughout the body), delirium, coma, organ failure, and death.[20] Organ failure is a result of the bacteria infecting organs through the bloodstream.[14] Other forms of the disease include septicemic plague and pneumonic plague, in which the bacterium reproduces in the person's blood and lungs respectively.[21]
Diagnosis
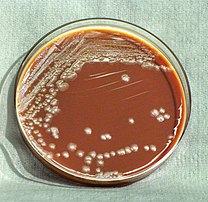
Laboratory testing is required in order to diagnose and confirm plague. Ideally, confirmation is through the identification of Y. pestis culture from a patient sample. Confirmation of infection can be done by examining serum taken during the early and late stages of infection. To quickly screen for the Y. pestis antigen in patients, rapid dipstick tests have been developed for field use.[22]
Samples taken for testing include:[23]
- Buboes: Swollen lymph nodes (buboes) characteristic of bubonic plague, a fluid sample can be taken from them with a needle.
- Blood: blood cultures test blood samples for bacteria to find source of infection[24]
- Lungs: Spirometry test are used to screen the lungs for diseases that effect the airways. Chest X-rays of the lungs are also used as an effective method of diagnosis.[25]
Prevention
Bubonic plague outbreaks are controlled by pest control and modern sanitation techniques. This disease uses fleas commonly found on rats as a vector to jump from animals to humans. The mortality rate is highest in the summer and early fall.[26] The successful control of rat populations in dense urban areas is essential to outbreak prevention. One example is the use of a machine called the Sulfurozador, used to deliver sulphur dioxide to eradicate the pest that spread the bubonic plague in Buenos Aires, Argentina during the early 18th century.[27] Targeted chemoprophylaxis, sanitation, and vector control also played a role in controlling the 2003 Oran outbreak of the bubonic plague.[28] Another means of prevention in large European cities was a city-wide quarantine to not only limit interaction with people who were infected, but also to limit the interaction with the infected rats.[29]
Treatment
Several classes of antibiotic are effective in treating bubonic plague. These include aminoglycosides such as streptomycin and gentamicin, tetracyclines (especially doxycycline), and the fluoroquinolone ciprofloxacin. Mortality associated with treated cases of bubonic plague is about 1–15%, compared to a mortality of 40–60% in untreated cases.[30]
People potentially infected with the plague need immediate treatment and should be given antibiotics within 24 hours of the first symptoms to prevent death. Other treatments include oxygen, intravenous fluids, and respiratory support. People who have had contact with anyone infected by pneumonic plague are given prophylactic antibiotics.[31] Using the broad-based antibiotic streptomycin has proven to be dramatically successful against the bubonic plague within 12 hours of infection.[32]
Epidemiology

Globally between 2010 and 2015, there were 3,248 documented cases, which resulted in 584 deaths.[1] The countries with the greatest number of cases are the Democratic Republic of the Congo, Madagascar, and Peru.[1]
For over a decade since 2001, Zambia, India, Malawi, Algeria, China, Peru, and the Democratic Republic of the Congo had the most plague cases, with over 1,100 cases in the Democratic Republic of the Congo alone. From 1,000 to 2,000 cases are conservatively reported per year to the WHO.[33] From 2012 to 2017, reflecting political unrest and poor hygienic conditions, Madagascar began to host regular epidemics.[33]
Between 1900 and 2015, the United States had 1,036 human plague cases, with an average of 9 cases per year. In 2015, 16 people in the western United States developed plague, including 2 cases in Yosemite National Park.[34] These US cases usually occur in rural northern New Mexico, northern Arizona, southern Colorado, California, southern Oregon, and far western Nevada.[35]
In November 2017, the Madagascar Ministry of Health reported an outbreak to the WHO (World Health Organization) with more cases and deaths than any recent outbreak in the country. Unusually, most of the cases were pneumonic rather than bubonic.[36]
In June 2018, a child was confirmed to be the first person in Idaho to be infected by bubonic plague in nearly 30 years.[37]
A couple died in May 2019, in Mongolia, while hunting marmots.[38] Another two people in the province of Inner Mongolia, China, were treated in November 2019 for the disease.[39]

In July 2020, in Bayannur, Inner Mongolia of China, a human case of bubonic plague was reported. Officials responded by activating a city-wide plague-prevention system for the remainder of the year.[40] Also in July 2020, in Mongolia, a teenager died from bubonic plague after consuming infected marmot meat.[41]
History
Yersinia pestis has been discovered in archaeological finds from the Late Bronze Age (~3800 BP).[42] The bacteria is identified by ancient DNA in human teeth from Asia and Europe dating from 2,800 to 5,000 years ago.[43] Some authors have suggested that the plague was responsible for the Neolithic decline.[44]
First pandemic
The first recorded epidemic affected the Sasanian Empire and their arch-rivals, the Eastern Roman Empire (Byzantine Empire) and was named the Plague of Justinian (541–549 AD) after emperor Justinian I, who was infected but survived through extensive treatment.[45][46] The pandemic resulted in the deaths of an estimated 25 million (6th century outbreak) to 50 million people (two centuries of recurrence).[47][48] The historian Procopius wrote, in Volume II of History of the Wars, of his personal encounter with the plague and the effect it had on the rising empire.
In the spring of 542, the plague arrived in Constantinople, working its way from port city to port city and spreading around the Mediterranean Sea, later migrating inland eastward into Asia Minor and west into Greece and Italy. The Plague of Justinian is said to have been "completed" in the middle of the 8th century.[15] Because the infectious disease spread inland by the transferring of merchandise through Justinian's efforts in acquiring luxurious goods of the time and exporting supplies, his capital became the leading exporter of the bubonic plague. Procopius, in his work Secret History, declared that Justinian was a demon of an emperor who either created the plague himself or was being punished for his sinfulness.[48]
Second pandemic


Medieval society's increasing population was put to deadly halt when, in the Late Middle Ages, Europe experienced the deadliest disease outbreak in history. They called it the Great Dying or The Great Pestilence, later coined The Black Death.[19] Lasting in potency for roughly 6 years, 1346–1352, the Black Death claimed one-third of the European human population. Having mortality rates as high as 70%-80%.[19] Some historians believe that society subsequently became more violent as the mass mortality rate cheapened life and thus increased warfare, crime, popular revolt, waves of flagellants, and persecution.[49] The Black Death originated in Central Asia and spread from Italy and then throughout other European countries. Arab historians Ibn Al-Wardni and Almaqrizi believed the Black Death originated in Mongolia. Chinese records also show a huge outbreak in Mongolia in the early 1330s.[50]
In 2022, researchers presented evidence that the plague originated near Lake Issyk-Kul in Kyrgyzstan.[51] The Mongols had cut the trade route (the Silk Road) between China and Europe, which halted the spread of the Black Death from eastern Russia to Western Europe. The European epidemic may have begun with the siege of Caffa, an attack that Mongols launched on the Italian merchants' last trading station in the region, Caffa, in the Crimea.[32]
In late 1346, plague broke out among the besiegers and from them penetrated the town. The Mongol forces catapulted plague-infested corpses into Caffa as a form of attack, one of the first known instances of biological warfare.[52] When spring arrived, the Italian merchants fled on their ships, unknowingly carrying the Black Death. Carried by the fleas on rats, the plague initially spread to humans near the Black Sea and then outwards to the rest of Europe as a result of people fleeing from one area to another. Rats migrated with humans, traveling among grain bags, clothing, ships, wagons, and grain husks.[20] Continued research indicates that black rats, those that primarily transmitted the disease, prefer grain as a primary meal.[15] Due to this, the major bulk grain fleets that transported major city's food shipments from Africa and Alexandria to heavily populated areas, and were then unloaded by hand, played a role in increasing the transmission effectiveness of the plague.[15]
Third pandemic
The plague resurfaced for a third time in the mid-19th century; this is also known as "the modern pandemic". Like the two previous outbreaks, this one also originated in Eastern Asia, most likely in Yunnan, a province of China, where there are several natural plague foci.[53] The initial outbreaks occurred in the second half of the 18th century.[54][55] The disease remained localized in Southwest China for several years before spreading. In the city of Canton, beginning in January 1894, the disease had killed 80,000 people by June. Daily water traffic with the nearby city of Hong Kong rapidly spread the plague there, killing over 2,400 within two months during the 1894 Hong Kong plague.[56]
The third pandemic spread the disease to port cities throughout the world in the second half of the 19th century and the early 20th century via shipping routes.[57] The plague infected people in Chinatown in San Francisco from 1900 to 1904,[58] and in the nearby locales of Oakland and the East Bay again from 1907 to 1909.[59] During the former outbreak, in 1902, authorities made permanent the Chinese Exclusion Act, a law originally signed into existence by President Chester A. Arthur in 1882. The Act was supposed to last for 10 years, but was renewed in 1892 with the Geary Act, then followed by the 1902 decision. The last major outbreak in the United States occurred in Los Angeles in 1924,[60] though the disease is still present in wild rodents and can be passed to humans that come in contact with them.[35] According to the World Health Organization, the pandemic was considered active until 1959, when worldwide casualties dropped to 200 per year. In 1994, a plague outbreak in five Indian states caused an estimated 700 infections (including 52 deaths) and triggered a large migration of Indians within India as they tried to avoid the disease.[citation needed]
It was during the 1894 Hong Kong plague outbreak that Alexandre Yersin isolated the bacterium responsible (Yersinia pestis),[61] a few days after Japanese bacteriologist Kitasato Shibasaburō had isolated it.[62][63] However, the latter's description was imprecise and also expressed doubts of its relation to the disease, and thus the bacterium is today only named after Yersin.[63][64]
Society and culture

The scale of death and social upheaval associated with plague outbreaks has made the topic prominent in many historical and fictional accounts since the disease was first recognized. The Black Death in particular is described and referenced in numerous contemporary sources, some of which, including works by Chaucer, Boccaccio, and Petrarch, are considered part of the Western canon. The Decameron, by Boccaccio, is notable for its use of a frame story involving individuals who have fled Florence for a secluded villa to escape the Black Death. First-person, sometimes sensationalized or fictionalized, accounts of living through plague years have also been popular across centuries and cultures. For example, Samuel Pepys's diary makes several references to his first-hand experiences of the Great Plague of London in 1665–66.[65]
Later works, such as Albert Camus's novel The Plague or Ingmar Bergman's film The Seventh Seal have used bubonic plague in settings, such as quarantined cities in either medieval or modern times, as a backdrop to explore a variety of concepts. Common themes include the breakdown of society, institutions, and individuals during the plague, the cultural and psychological existential confrontation with mortality, and the allegorical use of the plague about contemporary moral or spiritual questions.[citation needed]
Biological warfare
Some of the earliest instances of biological warfare were said to have been products of the plague, as armies of the 14th century were recorded catapulting diseased corpses over the walls of towns and villages to spread the pestilence. This was done by Jani Beg when he attacked the city of Kaffa in 1343.[66]
Later, plague was used during the Second Sino-Japanese War as a bacteriological weapon by the Imperial Japanese Army. These weapons were provided by Shirō Ishii's units and used in experiments on humans before being used in the field. For example, in 1940, the Imperial Japanese Army Air Service bombed Ningbo with fleas carrying the bubonic plague.[67] During the Khabarovsk War Crime Trials, the accused, such as Major General Kiyoshi Kawashima, testified that, in 1941, 40 members of Unit 731 air-dropped plague-contaminated fleas on Changde. These operations caused epidemic plague outbreaks.[29]
Continued research
Substantial research has been done regarding the origin of the plague and how it traveled through the continent.[15] Mitochondrial DNA of modern rats in Western Europe indicated that these rats came from two different areas, one being Africa and the other unclear.[15] The research regarding this pandemic has greatly increased with technology.[15] Through archaeo-molecular investigation, researchers have discovered the DNA of plague bacillus in the dental core of those that fell ill to the plague.[15] Analysis of teeth of the deceased allows researchers to further understand both the demographics and mortuary patterns of the disease. For example, in 2013 in England, archeologists uncovered a burial mound to reveal 17 bodies, mainly children, who had died of the Bubonic plague. They analyzed these burial remains using radiocarbon dating to determine they were from the 1530s, and dental core analysis revealed the presence of Yersinia pestis.[68]
Other evidence for rats that are currently still being researched consists of gnaw marks on bones, predator pellets and rat remains that were preserved in situ.[15] This research allows individuals to trace early rat remains to track the path traveled and in turn connect the impact of the Bubonic Plague to specific breeds of rats.[15] Burial sites, known as plague pits, offer archaeologists an opportunity to study the remains of people who died from the plague.[69]
Another research study indicates that these separate pandemics were all interconnected.[16] A current computer model indicates that the disease did not go away in between these pandemics.[16] It rather lurked within the rat population for years without causing human epidemics.[16]
See also
- List of cutaneous conditions
- List of epidemics
- Miasma theory
- Plague (disease)
- Plague doctor
- Hidradenitis suppurativa
References
- ^ a b c d e f g h i j k l m n o p q r s t u v w World Health Organization (November 2014). "Plague Fact sheet N°267". Archived from the original on 24 April 2015. Retrieved 10 May 2015.
- ^ a b "Plague Symptoms". 13 June 2012. Archived from the original on 19 August 2015. Retrieved 21 August 2015.
- ^ Clinic Bp. "Plague". Mayo Clinic. Retrieved 16 June 2022.
- ^ a b c d e f Prentice MB, Rahalison L (April 2007). "Plague". Lancet. 369 (9568): 1196–1207. doi:10.1016/S0140-6736(07)60566-2. PMID 17416264. S2CID 208790222.
- ^ a b "Plague Resources for Clinicians". 13 June 2012. Archived from the original on 21 August 2015. Retrieved 21 August 2015.
- ^ "Plague Ecology and Transmission". 13 June 2012. Archived from the original on 22 August 2015. Retrieved 21 August 2015.
- ^ Edman BF, Eldridge JD (2004). Medical Entomology a Textbook on Public Health and Veterinary Problems Caused by Arthropods (Rev. ed.). Dordrecht: Springer Netherlands. p. 390. ISBN 978-9400710092.
- ^ McMichael AJ (August 2010). "Paleoclimate and bubonic plague: a forewarning of future risk?". BMC Biology. 8 (1): 108. doi:10.1186/1741-7007-8-108. PMC 2929224. PMID 24576348.
- ^ Keyes DC (2005). Medical response to terrorism : preparedness and clinical practice. Philadelphia [u.a.]: Lippincott Williams & Wilkins. p. 74. ISBN 978-0781749862.
- ^ Haensch S, Bianucci R, Signoli M, Rajerison M, Schultz M, Kacki S, et al. (October 2010). "Distinct clones of Yersinia pestis caused the black death". PLOS Pathogens. 6 (10): e1001134. doi:10.1371/journal.ppat.1001134. PMC 2951374. PMID 20949072.
- ^ a b c "Plague History". 13 June 2012. Archived from the original on 21 August 2015. Retrieved 21 August 2015.
- ^ Cohn SK (2008). "Epidemiology of the Black Death and successive waves of plague". Medical History. Supplement. 52 (27): 74–100. doi:10.1017/S0025727300072100. PMC 2630035. PMID 18575083.
- ^ LeRoux N (2007). Martin Luther As Comforter: Writings on Death Volume 133 of Studies in the History of Christian Traditions. Brill. p. 247. ISBN 978-9004158801.
- ^ a b c d e Sebbane, Florent et al. "Kinetics of disease progression and host response in a rat model of bubonic plague." The American journal of pathology vol. 166:5 (2005)
- ^ a b c d e f g h i j k l m McCormick M (Summer 2003). "Rats, Communications, and Plague: Toward an Ecological History". The Journal of Interdisciplinary History. 34 (1): 1–25. doi:10.1162/002219503322645439. JSTOR 3656705. S2CID 128567627.
- ^ a b c d Travis J (21 October 2000). "Model Explains Bubonic Plague's Persistence". Society for Science and the Public.
- ^ a b "Symptoms of Plague". Brief Overview of Plague. iTriage. Healthagen, LLC. Archived from the original on 4 February 2016. Retrieved 26 November 2014.
- ^ Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. (May 2000). "Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense". JAMA. 283 (17): 2281–2290. doi:10.1001/jama.283.17.2281. PMID 10807389.
- ^ a b c Keeling MJ, Gilligan CA (2000). "Bubonic Plague: A Metapopulation Model of a Zoonosis". Proceedings: Biological Sciences. 267 (1458): 2219–2230. doi:10.1098/rspb.2000.1272. ISSN 0962-8452. JSTOR 2665900. PMC 1690796. PMID 11413636.
- ^ a b Echenberg M (2007). Plague Ports: The Global Urban Impact of Bubonic Plague, 1894–1901. New York: New York University Press. p. 9. ISBN 978-0814722336.
- ^ Byrne JP, ed. (2008). Encyclopedia of pestilence, pandemics, and plagues. Westport, Conn.: Greenwood Press. pp. 540–542. ISBN 978-0-313-34101-4. OCLC 226357719.
- ^ "Plague, Laboratory testing". Health Topics A to Z. Archived from the original on 17 November 2010. Retrieved 23 October 2010.
- ^ "Plague – Diagnosis and treatment – Mayo Clinic". www.mayoclinic.org. Retrieved 1 December 2017.
- ^ Gonzalez MD, Chao T, Pettengill MA (19 September 2020). "Modern Blood Culture". Clinics in Laboratory Medicine. 40 (4): 379–392. doi:10.1016/j.cll.2020.07.001. PMC 7501519. PMID 33121610.
- ^ Haynes JM (1 February 2018). "Basic Spirometry testing and interpretation for the primary care provider". Canadian Journal of Respiratory Therapy. 54 (4): 10.29390/cjrt–2018–017. doi:10.29390/cjrt-2018-017. PMC 6516140. PMID 31164790.
- ^ Theilmann J, Cate F (2007). "A Plague of Plagues: The Problem of Plague Diagnosis in Medieval England". The Journal of Interdisciplinary History. 37 (3): 374. JSTOR 4139605.
- ^ Engelmann L (July 2018). "Fumigating the Hygienic Model City: Bubonic Plague and the Sulfurozador in Early-Twentieth-Century Buenos Aires". Medical History. 62 (3): 360–382. doi:10.1017/mdh.2018.37. PMC 6113751. PMID 29886876.
- ^ Bertherat E, Bekhoucha S, Chougrani S, Razik F, Duchemin JB, Houti L, et al. (October 2007). "Plague reappearance in Algeria after 50 years, 2003". Emerging Infectious Diseases. 13 (10): 1459–1462. doi:10.3201/eid1310.070284. PMC 2851531. PMID 18257987.
- ^ a b Barenblatt D (2004). A Plague upon Humanity. pp. 220–221.
- ^ "Plague". Archived from the original on 5 March 2010. Retrieved 25 February 2010.
- ^ "Plague". Healthagen, LLC. Archived from the original on 6 June 2011. Retrieved 4 April 2011.
- ^ a b Echenberg, Myron (2002). Pestis Redux: The Initial Years of the Third Bubonic Plague Pandemic, 1894–1901. Journal of World History, vol 13,2
- ^ a b Filip J (11 April 2014). "Avoiding the Black Plague Today". The Atlantic. Retrieved 11 April 2014.
- ^ Regan M (January 2017). "Human Plague Cases Drop in the US". PBS. Retrieved 1 January 2017.
- ^ a b "Maps and Statistics: Plague in the United States". www.cdc.gov. 23 October 2017. Retrieved 1 December 2017.
- ^ "Emergencies Preparedness, Response, Plague, Madagascar, Disease Outbreak News | Plague | CDC". www.who.int. 15 November 2017. Archived from the original on 17 November 2017. Retrieved 15 November 2017.
- ^ "Elmore County Child Recovering After Plague Infection" (PDF). www.cdhd.idaho.gov. 12 June 2018. Retrieved 8 February 2023.
- ^ Schriber M, Bichell RE (7 May 2019). "Bubonic Plague Strikes in Mongolia: Why Is It Still A Threat?". NPR. Retrieved 8 May 2019.
- ^ Yeung J (14 November 2019). "Two people just got the plague in China – yes, the Black Death plague". CNN. Retrieved 14 November 2019.
- ^ Yeung J (7 July 2020). "Chinese authorities confirm case of bubonic plague in Inner Mongolia". CNN. Retrieved 6 July 2020.
- ^ Mathers M (14 July 2020). "Teenager dies of Black Death in Mongolia amid fears of new outbreak". The Independent. Retrieved 14 July 2020.
- ^ Spyrou MA, Tukhbatova RI, Wang CC, Valtueña AA, Lankapalli AK, Kondrashin VV, et al. (June 2018). "Analysis of 3800-year-old Yersinia pestis genomes suggests Bronze Age origin for bubonic plague". Nature Communications. 9 (1): 2234. Bibcode:2018NatCo...9.2234S. doi:10.1038/s41467-018-04550-9. PMC 5993720. PMID 29884871.
- ^ Rasmussen S, Allentoft ME, Nielsen K, Orlando L, Sikora M, Sjögren KG, et al. (October 2015). "Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago". Cell. 163 (3): 571–582. doi:10.1016/j.cell.2015.10.009. PMC 4644222. PMID 26496604.
- ^ Rascovan N, Sjögren KG, Kristiansen K, Nielsen R, Willerslev E, Desnues C, Rasmussen S (10 January 2019). "Emergence and Spread of Basal Lineages of Yersinia pestis during the Neolithic Decline". Cell. 176 (2): 295–305. doi:10.1016/j.cell.2018.11.005. PMID 30528431. S2CID 54447284.
- ^ Little LK (2007). "Life and Afterlife of the First Plague Pandemic.". In Little LK (ed.). Plague and the End of Antiquity: The Pandemic of 541–750. Cambridge University Press. pp. 8–15. ISBN 978-0-521-84639-4.
- ^ McCormick M (2007). "Toward a Molecular History of the Justinian Pandemic". In Little LK (ed.). Plague and the End of Antiquity: The Pandemic of 541–750. Cambridge University Press. pp. 290–312. ISBN 978-0-521-84639-4.
- ^ Rosen W (2007). Justinian's Flea: Plague, Empire, and the Birth of Europe. Viking Adult. p. 3. ISBN 978-0-670-03855-8. Archived from the original on 25 January 2010.
- ^ a b "The Plague of Justinian". History Magazine. 11 (1). Moorshead Magazines, Limited.: 9–12 2009 – via History Reference Center.
- ^ Cohn, Samuel K. (2002). "The Black Death: End of a Paradigm". American Historical Review, vol 107, 3, pp. 703–737
- ^ Sean Martin (2001). Black Death: Chapter One. Harpenden, GBR: Pocket Essentials. p. 14.[ISBN missing]
- ^ Spyrou MA, Musralina L, Gnecchi Ruscone GA, Kocher A, Borbone PG, Khartanovich VI, Buzhilova A, Djansugurova L, Bos KI, Kühnert D, Haak W, Slavin P, Krause J (2022). "The source of the Black Death in fourteenth-century central Eurasia". Nature. 606 (7915): 718–724. Bibcode:2022Natur.606..718S. doi:10.1038/s41586-022-04800-3. PMC 9217749. PMID 35705810.
- ^ Wheelis M (2002). "Biological Warfare at the 1346 Siege of Caffa". Emerging Infectious Diseases. 8 (9): 971–975. doi:10.3201/eid0809.010536. PMC 2732530. PMID 12194776.
- ^ Nicholas Wade (31 October 2010). "Europe's Plagues Came From China, Study Finds". The New York Times. Archived from the original on 4 November 2010. Retrieved 1 November 2010.
The great waves of plague that twice devastated Europe and changed the course of history had their origins in China, a team of medical geneticists reported Sunday, as did a third plague outbreak that struck less harmfully in the 19th century.
- ^ Benedict, Carol (1996). "Bubonic plague in nineteenth-century China". Modern China. 14 (2). Stanford, Calif.: Stanford Univ. Press: 107–155. doi:10.1177/009770048801400201. ISBN 978-0804726610. PMID 11620272. S2CID 220733020.
- ^ Cohn SK (2003). The Black Death Transformed: Disease and Culture in Early Renaissance Europe. A Hodder Arnold. p. 336. ISBN 978-0-340-70646-6.
- ^ Pryor EG (1975). "The great plague of Hong Kong" (PDF). Journal of the Hong Kong Branch of the Royal Asiatic Society. 15: 61–70. PMID 11614750. Archived (PDF) from the original on 16 January 2020. Retrieved 2 June 2014.
- ^ "History | Plague | CDC". www.cdc.gov. Retrieved 26 November 2017.
- ^ Porter D (11 September 2003). "Book Review". New England Journal of Medicine. 349 (11): 1098–1099. doi:10.1056/NEJM200309113491124. ISSN 0028-4793.
- ^ "On This Day: San Francisco Bubonic Plague Outbreak Begins". www.findingdulcinea.com. Archived from the original on 1 December 2017. Retrieved 25 November 2017.
- ^ Griggs MB. "30,000 People in Quarantine After Bubonic Plague Kills One in China". Smithsonian. Retrieved 25 November 2017.
- ^ Hong Kong Museum of Medical Sciences Society (2006). Plague, SARS and the Story of Medicine in Hong Kong. Hong Kong University Press. pp. 35–36. ISBN 978-962-209-805-3.
- ^ Pryor 1975, p. 66.
- ^ a b HKMSSS 2006, pp. 35–36.
- ^ Solomon T (1997). "Hong Kong, 1894: the role of James A Lowson in the controversial discovery of the plague bacillus". The Lancet. 350 (9070). Elsevier BV: 59–62 [61–62]. doi:10.1016/s0140-6736(97)01438-4. ISSN 0140-6736. PMID 9217728. S2CID 26567729.
- ^ Pepys S. "Samuel Pepys Diary – Plague extracts". Samuel Pepys Diary. Archived from the original on 7 April 2020. Retrieved 31 August 2019.
- ^ "Black Death –Origin and spread of the plague in Europe". www.britannica.com. Retrieved 10 October 2022.
- ^ "Japan triggered bubonic plague outbreak, doctor claims - Asia, World - the Independent". The Independent. Archived from the original on 12 September 2011.[dead link]
- ^ Pinkowski J (18 February 2020). "Black Death discovery offers rare new look at plague catastrophe". National Geographic. Retrieved 5 December 2020.
- ^ Antoine D (2008). "The archaeology of "plague"". Medical History. Supplement. 52 (27): 101–114. doi:10.1017/S0025727300072112. PMC 2632866. PMID 18575084.
Further reading
- Alexander JT (2003) [First published 1980]. Bubonic Plague in Early Modern Russia: Public Health and Urban Disaster. Oxford, UK; New York: Oxford University Press. ISBN 978-0-19-515818-2. OCLC 50253204.
- Batten-Hill D (2011). This Son of York. Kendal, England: David Batten-Hill. ISBN 978-1-78176-094-9. Archived from the original on 20 May 2013. Retrieved 27 August 2018.
- Biddle W (2002). A Field Guide to Germs (2nd Anchor Books ed.). New York: Anchor Books. ISBN 978-1-4000-3051-4. OCLC 50154403.
- Carol B (1996). Bubonic Plague in Nineteenth-Century China. Stanford, CA: Stanford University Press. ISBN 978-0-8047-2661-0. OCLC 34191853.
- Kool JL (April 2005). "Risk of person-to-person transmission of pneumonic plague". Clinical Infectious Diseases. 40 (8): 1166–72. doi:10.1086/428617. PMID 15791518.
- Little LK (2007). Plague and the End of Antiquity: The Pandemic of 541–750. New York: Cambridge University Press. ISBN 978-0-521-84639-4. OCLC 65361042.
- Rosen W (2007). Justinian's Flea: Plague, Empire and the Birth of Europe. London: Viking Penguin. ISBN 978-0-670-03855-8.
- Scott S, Duncan CJ (2001). Biology of Plagues: Evidence from Historical Populations. Cambridge, UK; New York: Cambridge University Press. ISBN 978-0-521-80150-8. OCLC 44811929.
External links
 Works related to An Account of the Plague at Constantinople at Wikisource
Works related to An Account of the Plague at Constantinople at Wikisource
