Lycopene: Difference between revisions
→Health effects: rm unnecessary subhead |
→Health effects: c/e, add Cochrane review finding insufficient evidence to make any conclusion regarding prostate |
||
| Line 192: | Line 192: | ||
==Health effects== |
==Health effects== |
||
| ⚫ | Given its potential properties in vivo, substantial research has been devoted to a possible correlation between lycopene consumption and general health. In 2005, the [[United States Food and Drug Administration]] allowed a limited, highly qualified claim to be used for tomatoes and tomato products which contain lycopene, as a guide that would not mislead consumers, namely: "Very limited and preliminary scientific research suggests that eating one-half to one cup of tomatoes and/or tomato sauce a week may reduce the risk of prostate cancer. FDA concludes that there is little scientific evidence supporting this claim."<ref name=fda>{{cite web | url=http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm073992.htm | title=Summary of Qualified Health Claims Subject to Enforcement Discretion | publisher=FDA | date=11 August 2005 | accessdate=25 September 2013}}</ref> A 2011 review found insufficient evidence to come to any conclusion about what effect lycopene might have on prostate symptoms, PSA levels or prostate cancer.<ref>{{Cite journal | last1 = Ilic | first1 = D. | last2 = Forbes | first2 = KM. | last3 = Hassed | first3 = C. | title = Lycopene for the prevention of prostate cancer. | journal = Cochrane Database Syst Rev | volume = | issue = 11 | pages = CD008007 | month = | year = 2011 | doi = 10.1002/14651858.CD008007.pub2 | PMID = 22071840 }}</ref> |
||
Given its potential properties in vivo, substantial research has been devoted to a possible correlation between lycopene consumption and general health. |
|||
| ⚫ | In 2005, the [[United States Food and Drug Administration]] allowed a limited, highly qualified claim to be used for tomatoes and tomato products which contain lycopene, as a guide that would not mislead consumers, namely: |
||
<!--==Food coloring== |
<!--==Food coloring== |
||
[[Image:Lycopene in DCM.jpg|thumb|50px|Lycopene, here dissolved in dichloromethane, is an intense bright red pigment.]]--> |
[[Image:Lycopene in DCM.jpg|thumb|50px|Lycopene, here dissolved in dichloromethane, is an intense bright red pigment.]]--> |
||
Revision as of 20:01, 25 September 2013

| |
| Names | |
|---|---|
| IUPAC name
ψ,ψ-Carotene
| |
| Other names
(6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)- 2,6,10,14,19,23,27,31-Octamethyldotriaconta-2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.227 |
| EC Number |
|
| E number | E160d (colours) |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C40H56 | |
| Molar mass | 536.888 g·mol−1 |
| Appearance | Deep red solid |
| Density | 0.889 g/mL |
| Insoluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Supplementary data page | |
| Lycopene (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lycopene (from the New Latin word lycopersicum, referring to the tomato species) is a bright red carotene and carotenoid pigment and phytochemical found in tomatoes and other red fruits and vegetables, such as red carrots, red bell peppers, watermelons, gac, and papayas (but not strawberries or cherries).[1] Although lycopene is chemically a carotene, it has no vitamin A activity.[2]
In plants, algae, and other photosynthetic organisms, lycopene is an important intermediate in the biosynthesis of many carotenoids, including beta carotene, responsible for yellow, orange or red pigmentation, photosynthesis, and photo-protection. Like all carotenoids, lycopene is a polyunsaturated hydrocarbon (an unsubstituted alkene). Structurally, it is a tetraterpene assembled from eight isoprene units, composed entirely of carbon and hydrogen, and is insoluble in water. Lycopene's eleven conjugated double bonds give it its deep red color and are responsible for its antioxidant activity. Due to its strong color and non-toxicity, lycopene is a useful food coloring (registered as E160d) and is approved for usage in the USA,[3] Australia and New Zealand (registered as 160d)[4] and the EU.[5]
Lycopene is not an essential nutrient for humans, but is commonly found in the diet, mainly from dishes prepared from tomatoes. When absorbed from the stomach, lycopene is transported in the blood by various lipoproteins and accumulates in the liver, adrenal glands, and testes.
Because preliminary research has shown an inverse correlation between consumption of tomatoes and cancer risk, lycopene has been considered a potential agent for prevention of some types of cancers, particularly prostate cancer.[2] However, this area of research and the relationship with prostate cancer have been deemed insufficient of evidence for health claim approval by the US Food and Drug Administration (see below under Antioxidant properties and potential health benefits).
 |
 |
Structure and physical properties
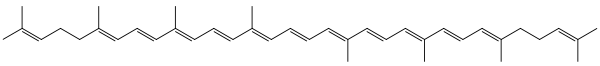
Lycopene is a symmetrical tetraterpene assembled from 8 isoprene units. It is a member of the carotenoid family of compounds, and because it consists entirely of carbon and hydrogen, is also a carotene.[6] Isolation procedures for lycopene were first reported in 1910, and the structure of the molecule was determined by 1931. In its natural, all-trans form, the molecule is long and straight, constrained by its system of eleven conjugated double bonds. Each extension in this conjugated system reduces the energy required for electrons to transition to higher energy states, allowing the molecule to absorb visible light of progressively longer wavelengths. Lycopene absorbs all but the longest wavelengths of visible light, so it appears red.[7]
Plants and photosynthetic bacteria naturally produce all-trans lycopene, but a total of 72 geometric isomers of the molecule are sterically possible.[8] When exposed to light or heat, lycopene can undergo isomerization to any of a number of these cis-isomers, which have a bent rather than linear shape. Different isomers were shown to have different stabilities due to their molecular energy (highest stability: 5-cis ≥ all-trans ≥ 9-cis ≥ 13-cis > 15-cis > 7-cis > 11-cis: lowest).[9] In the human bloodstream, various cis-isomers constitute more than 60% of the total lycopene concentration, but the biological effects of individual isomers have not been investigated.[10]
Staining and removal
Lycopene is insoluble in water, and can be dissolved only in organic solvents and oils. Because of its non-polarity, lycopene in food preparations will stain any sufficiently porous material, including most plastics. While a tomato stain can be fairly easily removed from fabric (provided the stain is fresh), lycopene diffuses into plastic, making it impossible to remove with hot water or detergent. If lycopene is oxidized (for example, by reacting with bleaches or acids), the double bonds between the carbon atoms will be broken; cleaving the molecule, breaking the conjugated double bond system, and eliminating the chromophore.
Role in photosynthesis

Carotenoids like lycopene are important pigments found in photosynthetic pigment-protein complexes in plants, photosynthetic bacteria, fungi, and algae. They are responsible for the bright colors of fruits and vegetables, perform various functions in photosynthesis, and protect photosynthetic organisms from excessive light damage. Lycopene is a key intermediate in the biosynthesis of many important carotenoids, such as beta-carotene, and xanthophylls.[11]
Biosynthesis
The unconditioned biosynthesis of lycopene in eukaryotic plants and in prokaryotic cyanobacteria is similar, as are the enzymes involved.[12] Synthesis begins with mevalonic acid, which is converted into dimethylallyl pyrophosphate. This is then condensed with three molecules of isopentenyl pyrophosphate (an isomer of dimethylallyl pyrophosphate), to give the twenty carbon geranylgeranyl pyrophosphate. Two molecules of this product are then condensed in a tail-to-tail configuration to give the forty carbon phytoene, the first committed step in carotenoid biosynthesis. Through several desaturation steps, phytoene is converted into lycopene. The two terminal isoprene groups of lycopene can be cyclized to produce beta carotene, which can then be transformed into a wide variety of xanthophylls.[13]
Dietary sources
| Dietary sources of lycopene[14] | |
|---|---|
| Source | μg/g wet weight |
| Gac | 2,000–2,300 |
| Raw tomato | 8.8–42 |
| Tomato juice | 86–100 |
| Tomato sauce | 63–131 |
| Tomato ketchup | 124 |
| Watermelon | 23–72 |
| Pink grapefruit | 3.6–34 |
| Pink guava | 54 |
| Papaya | 20–53 |
| Rosehip puree | 7.8 |
| Apricot | < 0.1 |
Fruits and vegetables that are high in lycopene include gac, tomatoes, watermelon, pink grapefruit, pink guava, papaya, seabuckthorn, wolfberry (goji, a berry relative of tomato), and rosehip. Although gac (Momordica cochinchinensis Spreng) has the highest content of lycopene of any known fruit or vegetable, up to 70 times more than tomatoes for example,[15] due to gac's rarity outside its native region of southeast Asia, tomatoes and tomato-based sauces, juices, and ketchup account for more than 85% of the dietary intake of lycopene for most people.[16] The lycopene content of tomatoes depends on species and increases as the fruit ripens.[17]
Unlike other fruits and vegetables, where nutritional content such as vitamin C is diminished upon cooking, processing of tomatoes increases the concentration of bioavailable lycopene. Lycopene in tomato paste is four times more bioavailable than in fresh tomatoes. For this reason, tomato paste is a preferable source as opposed to raw tomatoes.
While most green leafy vegetables and other sources of lycopene are low in fats and oils, lycopene is insoluble in water and is tightly bound to vegetable fiber. Processed tomato products such as pasteurized tomato juice, soup, sauce, and ketchup contain the highest concentrations of bioavailable lycopene from tomato-based sources.
Cooking and crushing tomatoes (as in the canning process) and serving in oil-rich dishes (such as spaghetti sauce or pizza) greatly increases assimilation from the digestive tract into the bloodstream. Lycopene is fat-soluble, so the oil is said to help absorption. Gac is a notable exception, containing high concentrations of lycopene and also saturated and unsaturated fatty acids.[18]
Lycopene may be obtained from vegetables and fruits such as the tomato, but another source of lycopene is the fungus Blakeslea trispora. Gac is a promising commercial source of lycopene for the purposes of extraction and purification.
The cis-lycopene from some varieties of tomato is more bioavailable.[19]
Note that there are some resources which make the mistaken assumption that all red fruits contain lycopene, when in fact many are pigmented by other chemicals. An example is the blood orange, which is colored by anthocyanin, while other red colored oranges, such as the Cara cara navel, and other citrus fruit, such as pink grapefruit, are colored by lycopene.
In addition, some foods which do not appear red also contain lycopene, e.g., asparagus, which contains about 30μg of lycopene per 100 gram serving[20] and dried parsley and basil, which contain about 3.5-7 μg of lycopene per gram[21]
Pharmacokinetics
| Distribution of lycopene[22] | |
|---|---|
| Tissue | nmol/g wet weight |
| Liver | 1.28–5.72 |
| Kidney | 0.15–0.62 |
| Adrenal | 1.9–21.6 |
| Testes | 4.34–21.4 |
| Ovary | 0.25–0.28 |
| Adipose | 0.2–1.3 |
| Lung | 0.22–0.57 |
| Colon | 0.31 |
| Breast | 0.78 |
| Skin | 0.42 |
After ingestion, lycopene is incorporated into lipid micelles in the small intestine. These micelles are formed from dietary fats and bile acids, and help to solubilize the hydrophobic lycopene and allow it to permeate the intestinal mucosal cells by a passive transport mechanism. Little is known about the liver metabolism of lycopene, but like other carotenoids, lycopene is incorporated into chylomicrons and released into the lymphatic system. In blood plasma, lycopene is eventually distributed into the very low and low density lipoprotein fractions.[23] Lycopene is mainly distributed to fatty tissues and organs such as the adrenal glands, liver, prostate and testes.
Adverse effects

Lycopene is non-toxic and is commonly found in the diet, but cases of excessive carotenoid intake have been reported. In a middle-aged woman who had prolonged and excessive consumption of tomato juice, her skin and liver were colored orange-yellow and she had elevated levels of lycopene in her blood. After three weeks on a lycopene-free diet her skin color returned to normal.[23] This discoloration of the skin is known as lycopenodermia[24] and is non-toxic.
There are also cases of intolerance or allergic reaction to dietary lycopene, which may cause diarrhea, nausea, stomach pain or cramps, gas, vomiting, and loss of appetite.[25]
Health effects
Given its potential properties in vivo, substantial research has been devoted to a possible correlation between lycopene consumption and general health. In 2005, the United States Food and Drug Administration allowed a limited, highly qualified claim to be used for tomatoes and tomato products which contain lycopene, as a guide that would not mislead consumers, namely: "Very limited and preliminary scientific research suggests that eating one-half to one cup of tomatoes and/or tomato sauce a week may reduce the risk of prostate cancer. FDA concludes that there is little scientific evidence supporting this claim."[26] A 2011 review found insufficient evidence to come to any conclusion about what effect lycopene might have on prostate symptoms, PSA levels or prostate cancer.[27]
See also
References
- ^ Meschino Health. "Comprehensive Guide to Lycopene". Retrieved 10 May 2012.
- ^ a b Journal of the American College of Nutrition: Role of Antioxidant Lycopene in Cancer and Heart Disease
- ^ 21 CFR 73.585
- ^ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ^ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
- ^ Grossman et al. (2004) p. 129
- ^ Rao et al. (2007) p. 210
- ^ 1054 isomers are theoretically possible, but only 72 are possible due to steric hinderance. IARC Handbook, (1998) p. 25
- ^ Chasse et al. Journal of Molecular Structure: THEOCHEM, Volume 571, Number 1, 27 August 2001 , pp. 27-37(11)[1]
- ^ Lycopene: Its role in human health and disease, Rao 'et al.', AGROFood industry hi-tech, July/August 2003 [2]
- ^ NDSU Agriculture. "What Color is Your Food?". Retrieved 10 May 2012.
- ^ Cunningham (2007) p. 533
- ^ Armstrong (1996) p. 229
- ^ Rao and Rao (2007) pp. 209–210
- ^ USDA study on Cartenoid content of gac fruit
- ^ Rao (2007) p.
- ^ Khan et al. (2008) p. 495
- ^ Ishida BK, Turner C, Chapman MH, McKeon TA (2004). "Fatty acid and carotenoid composition of gac (Momordica cochinchinensis Spreng) fruit". Journal of Agricultural and Food Chemistry. 52 (2): 274–9. doi:10.1021/jf030616i. PMID 14733508.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ http://www.medicalnewstoday.com/articles/64157.php
- ^ "Nutrition Facts Comparison Tool, Asparagus". Retrieved December 17, 2011.
- ^ "Nutrition Facts Comparison Tool, Spices Parsley, Basil". Retrieved December 17, 2011.
- ^ Stahl (1996) p. 7
- ^ a b Stahl (1996) p. 6
- ^ Institute of Medicine, Food and Nutrition Board. Beta-carotene and other carotenoids. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, D.C.: National Academy Press; 2000:325-400.
- ^ "Lycopene". Mayo Clinic. October 1, 2011. Retrieved December 17, 2011.
- ^ "Summary of Qualified Health Claims Subject to Enforcement Discretion". FDA. 11 August 2005. Retrieved 25 September 2013.
- ^ Ilic, D.; Forbes, KM.; Hassed, C. (2011). "Lycopene for the prevention of prostate cancer". Cochrane Database Syst Rev (11): CD008007. doi:10.1002/14651858.CD008007.pub2. PMID 22071840.
{{cite journal}}: Cite has empty unknown parameter:|month=(help)
Bibliography
- Armstrong GA, Hearst JE (1996). "Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis". FASEB J. 10 (2): 228–37. PMID 8641556.
- Basu A, Imrhan V (2007). "Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials". Eur J Clin Nutr. 61 (3): 295–303. doi:10.1038/sj.ejcn.1602510. PMID 16929242.
- Berneburg M, Grether-Beck S, Kurten V, Ruzicka T, Briviba K, Sies H, Krutmann J (1999). "Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion". The Journal of Biological Chemistry. 274 (22): 15345–15349. doi:10.1074/jbc.274.22.15345. PMID 10336420.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - Britton, George; Synnove Liaaen-Jensen; Hanspeter Pfander; (1996). Carotenoids : Synthesis (Carotenoids). Boston: Birkhauser. ISBN 3-7643-5297-3.
{{cite book}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - Cunningham FX, Lee H, Gantt E (2007). "Carotenoid biosynthesis in the primitive red alga Cyanidioschyzon merolae". Eukaryotic Cell. 6 (3): 533–45. doi:10.1128/EC.00265-06. PMC 1828917. PMID 17085635.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Di Mascio P, Kaiser S, Sies H (1989). "Lycopene as the most efficient biological carotenoid singlet oxygen quencher". Arch. Biochem. Biophys. 274 (2): 532–8. doi:10.1016/0003-9861(89)90467-0. PMID 2802626.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Gerster H (1997). "The potential role of lycopene for human health". J Am Coll Nutr. 16 (2): 109–26. PMID 9100211.
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1995). "Intake of carotenoids and retinol in relation to risk of prostate cancer". J. Natl. Cancer Inst. 87 (23): 1767–76. doi:10.1093/jnci/87.23.1767. PMID 7473833.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Grossman AR, Lohr M, Im CS (2004). "Chlamydomonas reinhardtii in the landscape of pigments". Annu. Rev. Genet. 38 (1): 119–73. doi:10.1146/annurev.genet.38.072902.092328. PMID 15568974.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - IARC Working Group on the Evaluation of Cancer Preventive Agents (1998). IARC Handbooks of Cancer Prevention: Volume 2: Carotenoids (IARC Handbooks of Cancer Prevention). Oxford University Press, USA. p. 25. ISBN 92-832-3002-7.
- Khan N, Afaq F, Mukhtar H (2008). "Cancer chemoprevention through dietary antioxidants: progress and promise". Antioxid. Redox Signal. 10 (3): 475–510. doi:10.1089/ars.2007.1740. PMID 18154485.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rao AV, Rao LG (2007). "Carotenoids and human health". Pharmacol. Res. 55 (3): 207–16. doi:10.1016/j.phrs.2007.01.012. PMID 17349800.
{{cite journal}}: Unknown parameter|month=ignored (help) - Stahl W, Sies H (1996). "Lycopene: a biologically important carotenoid for humans?". Arch. Biochem. Biophys. 336 (1): 1–9. doi:10.1006/abbi.1996.0525. PMID 8951028.
- Giovannucci E, Willett WC, Stampfer MJ, Liu Y, Rimm EB (2002). "A prospective study of tomato products, lycopene, and prostate cancer risk". J. Natl Cancer Inst. 94 (5): 391–396. doi:10.1093/jnci/94.5.391.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Levy J, Sharoni Y, Danilenko M, Miinster A, Bosin E, Giat Y, Feldman B (1995). "Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene". Nutr Cancer. 24 (3): 257–266. doi:10.1080/01635589509514415. PMID 8610045.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Pollack A, Madar Z, Eisner Z, Nyska A, Oren,P (1997). "Inhibitory effect of lycopene on cataract development in galactosemic rats". Metab Pediatr Syst Ophthalmol. 19 (20): 31–36.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Nahum A, Sharoni Y, Prall OW, Levy J, Hirsch K, Watts CK, Danilenko M (2001). "Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27(Kip1) in the cyclin E-cdk2 complexes". Oncogene. 20 (26): 3428–436. doi:10.1038/sj.onc.1204452. PMID 11423993.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Narisawa T, Fukaura Y, Hasebe M, Ito M, Nishino H, Khachik F, Murakoshi M, Uemura S, Aizawa R (1996). "Ihibitory effects of natural carotenoids, alpha-carotene, beta-carotene, lycopene and lutein, on colonic aberrant crypt foci formation in rats". Cancer Lett. 107 (1): 137–142. doi:10.1016/0304-3835(96)04354-6. PMID 8913278.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- Phytochemicals as Nutraceuticals-Lycopene
- USDA Webpage on Lycopene Content of Gac - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit.
- Food Sources of Lycopene - Based on USDA (US Department of Agriculture) National Nutrient Database Release 21 (SR21).

