Aleglitazar
Appearance
| |
| Names | |
|---|---|
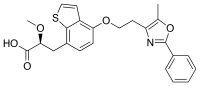
| Preferred IUPAC name
(2S)-2-Methoxy-3-{4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]-1-benzothiophen-7-yl}propanoic acid | |
| Other names
Ro-0728804, R-1439
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.220.523 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C24H23NO5S | |
| Molar mass | 437.51 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aleglitazar is a peroxisome proliferator-activated receptor agonist (hence a PPAR modulator ) with affinity to PPARα and PPARγ, which was under development by Hoffmann–La Roche for the treatment of type II diabetes.[1] It is no longer in phase III clinical trials.[2]
References
- ^ "Statement on a nonproprietary name adopted by the USAN Council: Aleglitazar" (PDF). United States Adopted Names. American Medical Association. Retrieved 2008-08-17.
- ^ "Roche halts diabetes drug trial in blow to pipeline". Roche. 2013-07-10. Retrieved 2013-07-10.
