Ramoplanin
 Structure of Ramoplanin A2, with the variable acyl sidechain in blue. | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.161.388 |
| Chemical and physical data | |
| Formula | C119H154ClN21O40 |
| Molar mass | 2554.10 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ramoplanin (INN) is a glycolipodepsipeptide antibiotic drug derived from strain ATCC 33076 of Actinoplanes.[1] It is effective against Gram-positive bacteria.[2]
Mechanism
It exerts its bacteriocidal effect by inhibiting cell wall biosynthesis, acting by inhibiting the transglycosylation step of peptidoglycan synthesis.[3] Ramoplanin specifically binds to and sequesters lipid intermediates I and II, preventing intracellular glycosyltransferase (MurG) and other steps of the peptidoglycan assembly system.[4]
Uses
Its development has been fast-tracked by the U.S. Food and Drug Administration as a treatment for multiple antibiotic-resistant Clostridium difficile infection of the gastrointestinal tract,[5] Unlike vancomycin, it is absorbed in the gastrointestinal tract, although it is unstable in the bloodstream, so can be taken only orally against Clostridium difficile infections of the gastrointestinal tract.[6][7][8]
Ramoplanin is "particularly useful" in cases E. faecalis no matter its sensitivity to vancomycin.[2]
Chemistry
Rapoplanin is a mixture of three related compounds that vary in the acyl group on the Asn N-terminus, with the most abundant form (shown in the infobox) being A2.[4]: Fig. 1
Synthesis
Biosynthesis
The biosynthesis is performed by a 33-gene cluster containing nonribosomal peptide synthetase genes and supporting enzymes for amino acid and fatty acid synthesis, revealed by sequencing of the producer strain in 2002. Initial investigation into the functions of individual genes were conducted in 2012.[9]
Total synthesis
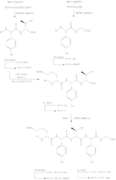
The general synthesis of Ramoplanin A1, A2 and A3 aglycons entails the preparation of residues 3-9 (heptapeptide 15), pentadepsipeptide 26 (residues 1, 2 and 15–17) along with pentapeptide 34 (residues 10–14), subsequent coupling, and cyclization to create the 49-membered aglycon core of the compound.[10] The synthesis of Ramoplanin A2 aglycon and A3 aglycon are very similar to scheme 6, where ramoplanin A1 aglycon requires the corresponding acyl group and DMF, while ramoplanin A3 aglycon synthesis requires both Bu
4NF, i-PrOH, and then treatment with the acyl group and DMF.[4]
- Ramoplanin A2 Aglycon Synthesis
-
1 - Synthesis of Hpg3-Phe9 Subunit
-
2 - Fmoc-L-HAsn(Trt)-Obn Preparation
-
3 - Synthesis of Depsipeptide subunit
-
4 - Synthesis of Orn10-Gly14 Subunit
-
5 - Preparation of acyl group
-
6 - Deprotection
References
- ^ Farver DK, Hedge DD, Lee SC (May 2005). "Ramoplanin: a lipoglycodepsipeptide antibiotic". The Annals of Pharmacotherapy. 39 (5): 863–8. doi:10.1345/aph.1E397. PMID 15784805. S2CID 6026698.
- ^ a b Lee, V. J. (2007-01-01), Taylor, John B.; Triggle, David J. (eds.), "7.22 - Anti-Gram Positive Agents of Natural Product Origins", Comprehensive Medicinal Chemistry II, Oxford: Elsevier, pp. 653–671, doi:10.1016/b0-08-045044-x/00222-4, ISBN 978-0-08-045044-5, retrieved 2022-06-04
- ^ Fang X, Tiyanont K, Zhang Y, Wanner J, Boger D, Walker S (January 2006). "The mechanism of action of ramoplanin and enduracidin". Molecular BioSystems. 2 (1): 69–76. doi:10.1039/b515328j. PMID 16880924.
- ^ a b c Shin, Dongwoo; Rew, Yosup; Boger, Dale L. (2004-08-17). "Total synthesis and structure of the ramoplanin A1 and A3 aglycons: Two minor components of the ramoplanin complex". Proceedings of the National Academy of Sciences. 101 (33): 11977–11979. doi:10.1073/pnas.0401419101. ISSN 0027-8424. PMC 514419. PMID 15175429.
- ^ Fulco P, Wenzel RP (December 2006). "Ramoplanin: a topical lipoglycodepsipeptide antibacterial agent". Expert Review of Anti-Infective Therapy. 4 (6): 939–45. doi:10.1586/14787210.4.6.939. PMID 17181409. S2CID 12881565.
- ^ Scheinfeld N (January 2007). "A comparison of available and investigational antibiotics for complicated skin infections and treatment-resistant Staphylococcus aureus and enterococcus". Journal of Drugs in Dermatology. 6 (1): 97–103. PMID 17373167.
- ^ Balagopal A, Sears CL (October 2007). "Clostridium difficile: new therapeutic options". Current Opinion in Pharmacology. 7 (5): 455–8. doi:10.1016/j.coph.2007.05.007. PMID 17644040.
- ^ Gerding DN, Muto CA, Owens RC (January 2008). "Treatment of Clostridium difficile infection". Clinical Infectious Diseases. 46 Suppl 1: S32-42. doi:10.1086/521860. PMID 18177219.
- ^ Hoertz, AJ; Hamburger, JB; Gooden, DM; Bednar, MM; McCafferty, DG (15 January 2012). "Studies on the biosynthesis of the lipodepsipeptide antibiotic Ramoplanin A2". Bioorganic & Medicinal Chemistry. 20 (2): 859–65. doi:10.1016/j.bmc.2011.11.062. PMID 22222159.
- ^ Jiang, Wanlong; Wanner, Jutta; Lee, Richard J.; Bounaud, Pierre-Yves; Boger, Dale L. (2003-02-19). "Total Synthesis of the Ramoplanin A2 and Ramoplanose Aglycon". Journal of the American Chemical Society. 125 (7): 1877–1887. doi:10.1021/ja0212314. ISSN 0002-7863. PMID 12580615.