Cefuroxime
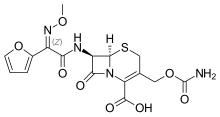 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zinacef, Ceftin, Furacia, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601206 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular, intravenous, by mouth |
| Drug class | Second-generation cephalosporin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 37% on an empty stomach, up to 52% if taken after food |
| Elimination half-life | 80 minutes |
| Excretion | Urine 66–100% unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.127 |
| Chemical and physical data | |
| Formula | C16H16N4O8S |
| Molar mass | 424.38 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cefuroxime, sold under the brand name Zinacef among others, is a second-generation cephalosporin[2] antibiotic used to treat and prevent a number of bacterial infections.[3] These include pneumonia, meningitis, otitis media, sepsis, urinary tract infections, and Lyme disease.[4] It is used by mouth or by injection into a vein or muscle.[4]
Common side effects include nausea, diarrhea, allergic reactions, and pain at the site of injection.[4] Serious side effects may include Clostridium difficile infection, anaphylaxis, and Stevens–Johnson syndrome.[4] Use in pregnancy and breastfeeding is believed to be safe.[5] It is a second-generation cephalosporin and works by interfering with a bacteria's ability to make a cell wall resulting in its death.[4]
Cefuroxime was patented in 1971, and approved for medical use in 1977.[6] It is on the World Health Organization's List of Essential Medicines.[7] In 2020, it was the 325th most commonly prescribed medication in the United States, with more than 800 thousand prescriptions.[8]
Medical uses[edit]
Cefuroxime is active against many bacteria including susceptible strains of Staphylococci and Streptococci, as well as a range of gram negative organisms.[9] As with the other cephalosporins, it is susceptible to beta-lactamase, although as a second-generation variety, it is less so. Hence, it may have greater activity against Haemophilus influenzae, Neisseria gonorrhoeae, and Lyme disease. Unlike other second-generation cephalosporins, cefuroxime can cross the blood–brain barrier.[10]
A systematic review found high quality evidence that injecting the eye with cefuroxime after cataract surgery will lower the chance of developing endophthalmitis after surgery.[11]
Side effects[edit]
Cefuroxime is generally well tolerated, and its side effects are usually transient. If ingested after food, this antibiotic is both better absorbed and less likely to cause its most common side effects of diarrhea, nausea, vomiting, headaches/migraines, dizziness, and abdominal pain compared to most antibiotics in its class.[medical citation needed]
Although a widely stated cross-allergic risk of about 10% exists between cephalosporins and penicillin, an assessment in 2006 have shown no increased risk for a cross-allergic reaction for cefuroxime and several other second-generation or later cephalosporins.[12]
Related compounds[edit]
Cefuroxime axetil is an acetoxyethyl ester prodrug of cefuroxime which is effective when taken by mouth.[13] It is a second-generation cephalosporin.[14]
Trade names[edit]
In the U.S. it is marketed as Zinacef by Covis Pharmaceuticals since the company acquired the U.S. rights to the product from GSK.[15] GSK had continued marketing a pediatric oral suspension as Ceftin; however, this presentation was discontinued as of 24 June 2017.[16]
In Bangladesh, it is available as Kilbac by Incepta, Axim by Aristopharma, Rofurox by Radiant and Xorimax by Sandoz. In India, it is available as Ceftum and "Cefuall" by Allencia Biosciences in tablet form and Supacef in injection form by GSK.[17] In Poland, it is available as Zamur by Mepha, subsidiary of Teva Pharmaceutical Industries.[18] In Australia, the "first generic" form of Cefuroxime axetil, Pharmacor Cefuroxime (tablets) from Pharmacor Pty Ltd, was registered on 27 March 2017, by the Therapeutic Goods Administration.[19] Cefuroxime axetil is sold in tablet form in Turkey inder the brand names Aksef [20] and Cefaks.[21] Cefuroxime axetil is also available (in two strengths) as granules for oral suspension from Aspen Pharmacare Australia Pty Ltd under the brand name Zinnat cefuroxime.[22]
References[edit]
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Katzung B (2018). Basic & Clinical Pharmacology. McGraw Hill. p. 803.
- ^ British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 518. ISBN 9780857113382.
- ^ a b c d e "Cefuroxime Sodium Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 22 March 2019.
- ^ "Cefuroxime Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 493. ISBN 9783527607495.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Cefuroxime – Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ "Appendix 5 – Antibiotic overview". Wellington ICU Drug Manual. Retrieved 7 April 2023.
- ^ Root RK, Waldvogel F, Corey L, Stamm WE (1999). Clinical Infectious Diseases: A Practical Approach. Oxford University Press. p. 259. ISBN 9780195081039.
- ^ Gower EW, Lindsley K, Tulenko SE, Nanji AA, Leyngold I, McDonnell PJ (February 2017). "Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery". The Cochrane Database of Systematic Reviews. 2017 (2): CD006364. doi:10.1002/14651858.CD006364.pub3. PMC 5375161. PMID 28192644.
- ^ Pichichero ME (February 2006). "Cephalosporins can be prescribed safely for penicillin-allergic patients" (PDF). The Journal of Family Practice. 55 (2): 106–112. PMID 16451776. Archived from the original (PDF) on 24 February 2012.
- ^ Sneader W (23 June 2005). Drug Discovery: History. John Wiley & Sons. ISBN 9780471899792.
- ^ Glatt AE (15 March 1986). "Second-generation cephalosporins". Hospital Practice (Office Ed.). 21 (3): 158A–158B, 158E, 158H–158L. doi:10.1080/21548331.1986.11704945. ISSN 8750-2836. PMID 3081544.
- ^ "Covis Pharma to Acquire U.S. Rights from GlaxoSmithKline for Fortaz®, Zinacef®, Lanoxin®, Parnate®, and Zantac® Injection". Covis-pharma-sarl (Press release). Retrieved 6 August 2012.
- ^ "FDA Drug Shortages". Food and Drug Administration (FDA). 20 March 2018.
- ^ "GlaxoSmithKline Pharmaceuticals Limited – Prescription Medicines – Anti-Infective". Gsk-india.com. 26 March 2013. Archived from the original on 22 March 2016. Retrieved 12 March 2012.
- ^ "Charakterystyka produktu lecznicznego" (PDF). Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych. 12 November 2015.
- ^ "Prescription medicines: registration of new generic medicines and biosimilar medicines, 2017". TGA. Therapeutic Goods Administration. 26 March 2018. Retrieved 30 July 2018.
- ^ "Aksef 500mg 20 Tablet". Nobel İlaç Sanayii ve Ticaret A.Ş. Retrieved 26 April 2023.
- ^ "Antiinfectives and antiparasitic products for systemic use". DEVA Holding. Retrieved 3 May 2023.
- ^ "ARTG ID 81301". TGA. Therapeutic Goods Administration. Archived from the original on 28 August 2021. Retrieved 30 July 2018.
External links[edit]
- "Cefuroxime". Drug Information Portal. U.S. National Library of Medicine.
