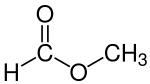
Methyl formate
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methyl formate | |||
| Systematic IUPAC name
Methyl methanoate | |||
| Other names
R-611
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.166 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C2H4O2 | |||
| Molar mass | 60.052 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | pleasant[1] | ||
| Density | 0.98 g/cm3 | ||
| Melting point | −100 °C (−148 °F; 173 K) | ||
| Boiling point | 32 °C (90 °F; 305 K) | ||
| 30% (20°C)[1] | |||
| Vapor pressure | 634 hPa (476 mmHg) (20°C)[1] | ||
| -32.0·10−6 cm3/mol | |||
| Hazards | |||
| GHS labelling:[3] | |||
 
| |||
| Danger | |||
| H224, H302, H319, H332, H335 | |||
| P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P312, P330, P337+P313, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −19 °C; −2 °F; 254 K[1] | ||
| Explosive limits | 4.5%-23%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
1622 mg/kg (oral, rabbit)[2] | ||
LCLo (lowest published)
|
50,000 ppm (guinea pig, 20 min)[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 100 ppm (250 mg/m3)[1] | ||
REL (Recommended)
|
TWA 100 ppm (250 mg/m3) ST 150 ppm (375 mg/m3)[1] | ||
IDLH (Immediate danger)
|
4500 ppm[1] | ||
| Safety data sheet (SDS) | Oxford MSDS | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.[4]
Production
In the laboratory, methyl formate can be produced by the condensation reaction of methanol and formic acid, as follows:
- HCOOH + CH3OH → HCOOCH3 + H2O
Industrial methyl formate, however, is usually produced by the combination of methanol and carbon monoxide (carbonylation) in the presence of a strong base, such as sodium methoxide:[4]

This process, practiced commercially by BASF among other companies gives 96% selectivity toward methyl formate. The catalyst for this process is sensitive to water, which can be present in the carbon monoxide feedstock, which is commonly derived from synthesis gas. Very dry carbon monoxide is, therefore, essential.[5]
Uses
Methyl formate is used primarily to manufacture formamide, dimethylformamide, and formic acid. These compounds are precursors or building blocks for many useful derivatives.
Because of its high vapor pressure, it is used for quick-drying finishes and as a blowing agent for some polyurethane foam applications (for example Ecomate® manufactured by Foam Supplies Inc.) and as a replacement for CFCs, HCFCs, and HFCs. Methyl formate has zero ozone depletion potential and zero global warming potential[citation needed]. It is also used as an insecticide.
A historical use of methyl formate, which sometimes brings it attention, was in refrigeration. Before the introduction of less-toxic refrigerants, methyl formate was used as an alternative to sulfur dioxide in domestic refrigerators, such as some models of the famous GE Monitor Top.
References
- ^ a b c d e f g h NIOSH Pocket Guide to Chemical Hazards. "#0417". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Methyl formate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Methyl formate". pubchem.ncbi.nlm.nih.gov. Retrieved 19 December 2021.
- ^ a b Werner Reutemann and Heinz Kieczka "Formic Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_013
- ^ W. Couteau, J. Ramioulle, US Patent US4216339