Titanium(IV) nitrate
| |
| Names | |
|---|---|
| Other names
titanium tetranitrate, tetranitratotitanium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.222.601 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Ti(NO3)4 | |
| Molar mass | 295.8866 g/mol |
| Appearance | white volatile solid |
| Density | 2.192[1] |
| Melting point | 58.5 °C (137.3 °F; 331.6 K) |
| Boiling point | decompose |
| Soluble | |
| Structure[2] | |
| monoclinic | |
| P21/C | |
a = 7.80, b = 13.57, c = 10.34 Å α = 90°, β = 125·0°, γ = 90°
| |
Lattice volume (V)
|
896.52 Å3 |
Formula units (Z)
|
4 |
| 8 | |
| flattened tetrahedral | |
| Related compounds | |
Related compounds
|
hafnium nitrate, zirconium nitrate, titanium phosphate, titanium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
Preparation
Similarly to its original method,[3][4] Ti(NO3)4 is prepared by the nitration of titanium tetrachloride using dinitrogen pentoxide:[5]
- TiCl4 + 4 N2O5 → Ti(NO3)4 + 4 ClNO2
A hydrated titanium nitrate is produced upon dissolution of titanium compounds in nitric acid.[6]
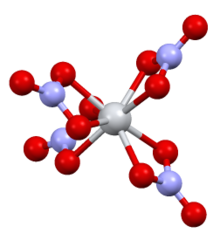
Structure
The complex has D2d symmetry, with four bidentate nitrate ligands. The N-O distances are 1·29 Å and 1·185 Å (noncoordinated).[2]
Physical properties
In the infrared spectrum, it absorbs strongly at 1635 cm−1, assigned to a N-O vibrational mode.[7]
It is soluble in nonpolar solvents silicon tetrachloride and carbon tetrachloride.[8][4]
Reactions
Titanium nitrate is hygroscopic, converting to ill-defined hydrates.[9] The anhydrous material is highly reactive, even toward hydrocarbons.[9] Titanium nitrate also reacts with n-dodecane,[10] p-dichlorobenzene, anisole, biphenyl,[10][11]
It decomposes thermally to titanium dioxide.[12]
References
- ^ "Titanium(iv) nitrate (Ti(NO3)4)". Retrieved 27 September 2014.
- ^ a b Garner, C. David; Ian H. Hillier; Martyn F. Guest (1975). "Ab initio self-consistent field molecular-orbital calculation of the ground state of tetranitratotitanium(IV); comments on the reactivity of anhydrous metal nitrates". Journal of the Chemical Society, Dalton Transactions (19): 1934. doi:10.1039/DT9750001934. ISSN 0300-9246.
- ^ Reihlen, Hans; Andreas Hake (1927). "Über die Konstitution des N2O4 und N2O3 und die Additionsverbindungen von Nitro- und Nitrosokörpern an Zinn- und Titantetrachlorid". Justus Liebig's Annalen der Chemie (in German). 452 (1): 47–67. doi:10.1002/jlac.19274520104. ISSN 0075-4617.
- ^ a b Schmeisser, Martin (1955). "Die Chemie der anorganischen Acylnitrate (ein Problem des Nitrylchlorids) und Acylperchlorate (ein Problem des Dichlorhexoxyds)". Angewandte Chemie (in German). 67 (17–18): 493–501. doi:10.1002/ange.19550671708. ISSN 0044-8249.
- ^ P. Ehrlich "Titanium Tetranitrate" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1237.
- ^ Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. Academic Press. p. 1331. ISBN 9780123526519. Retrieved 28 September 2014.
- ^ C. C. Addison, N. Logan, S. C. Wallwork and C. D. Garner, "Structural Aspects of Coordinated Nitrate Groups" Quart. Rev., Chem. Soc., 1971, volume 25, 289-322. doi:10.1039/qr9712500289.
- ^ Amos, D.W.; G.W. Flewett (1974). "Raman spectra of titanium (IV) and tin (IV) nitrates". Spectrochimica Acta Part A: Molecular Spectroscopy. 30 (2): 453–461. Bibcode:1974AcSpA..30..453A. doi:10.1016/0584-8539(74)80085-1. ISSN 0584-8539.
- ^ a b Amos, D.W.; D.A. Baines, G.W. Flewett (1973). "Nitration by titanium (IV) nitrate". Tetrahedron Letters. 14 (34): 3191–3194. doi:10.1016/S0040-4039(00)79808-X. ISSN 0040-4039.
- ^ a b Coombes, Robert G.; Leslie W. Russell (1974). "Nitration of aromatic compounds by tetranitratotitanium(IV) in carbon tetrachloride solution". Journal of the Chemical Society, Perkin Transactions 2 (7): 830. doi:10.1039/P29740000830. ISSN 0300-9580.
- ^ Schofield, Kenneth (1980). Aromatic Nitration. CUP Archive. pp. 97–98. ISBN 9780521233620. Retrieved 27 September 2014.
- ^ Allendorf, Mark Donald (1999-01-01). "Titanium Oxide CVD from Titanium (IV) Nitrate ...". Proceedings of the Symposium on Fundamental Gas-Phase and Surface Chemistry of Vapor-Phase Materials Synthesis. The Electrochemical Society. pp. 395–397. ISBN 9781566772174. Retrieved 27 September 2014.
Other reading
- Partington, J. R.; A. L. Whynes (1949). "660. Reactions of nitrosyl chloride. Part II". Journal of the Chemical Society (Resumed): 3135. doi:10.1039/JR9490003135. ISSN 0368-1769.
- Dauerman, L.; G.E. Salser (1973). "Mass spectra of covalent inorganic nitrates: copper(II) nitrate and titanium(IV) nitrate". Journal of Inorganic and Nuclear Chemistry. 35 (1): 304–306. doi:10.1016/0022-1902(73)80643-8. ISSN 0022-1902.
