Nitrite

| |||
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
dioxidonitrate(1−) | |||
| Other names
nitrite
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| NO− 2 | |||
| Molar mass | 46.005 g·mol−1 | ||
| Conjugate acid | Nitrous acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
The nitrite ion, which has the chemical formula NO−
2. Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries.[1] Nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite can also refer to organic compounds with the -ONO group, which are esters of nitrous acid.
Production
Sodium nitrite is made industrially by passing "nitrous fumes" into aqueous sodium hydroxide or sodium carbonate solution:[2][1]
The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid:
- 2 NH3 + H2O + N2O3 → 2 NH4NO2
Structure

2, which contribute to the resonance hybrid for the nitrite ion.
The nitrite ion has a symmetrical structure (C2v symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is described as a resonance hybrid with equal contributions from two canonical forms that are mirror images of each other. In molecular orbital theory, there is a sigma bond between each oxygen atom and the nitrogen atom, and a delocalized pi bond made from the p orbitals on nitrogen and oxygen atoms which is perpendicular to the plane of the molecule. The negative charge of the ion is equally distributed on the two oxygen atoms. Both nitrogen and oxygen atoms carry a lone pair of electrons. Therefore, the nitrite ion is a Lewis base.
Reactions
Acid-base properties
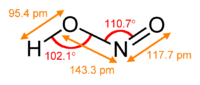
Nitrite is the conjugate base of the weak acid nitrous acid:
Nitrous acid is also highly volatile – in the gas phase it exists predominantly as a trans-planar molecule. In solution, it is unstable with respect to the disproportionation reaction:
- 3HNO2 (aq) ⇌ H3O+ + NO−
3 + 2NO
This reaction is slow at 0 °C.[2] Addition of acid to a solution of a nitrite in the presence of a reducing agent, such as iron(II), is a way to make nitric oxide (NO) in the laboratory.
Oxidation and reduction
The formal oxidation state of the nitrogen atom in nitrite is +3. This means that it can be either oxidized to oxidation states +4 and +5, or reduced to oxidation states as low as −3. Standard reduction potentials for reactions directly involving nitrous acid are shown in the table below:[4]
Half-reaction E0 (V) NO−
3 + 3 H+ + 2 e− ⇌ HNO2 + H2O+0.94 2 HNO2 + 4 H+ + 4 e− ⇌ H2N2O2 + 2 H2O +0.86 N2O4 + 2 H+ + 2 e− ⇌ 2 HNO2 +1.065 2 HNO2+ 4 H+ + 4 e− ⇌ N2O + 3 H2O +1.29
The data can be extended to include products in lower oxidation states. For example:
- H2N2O2 + 2 H+ + 2 e− ⇌ N2 + 2 H2O; E0 = +2.65 V
Oxidation reactions usually result in the formation of the nitrate ion, with nitrogen in oxidation state +5. For example, oxidation with permanganate ion can be used for quantitative analysis of nitrite (by titration):
- 5 NO−
2 + 2 MnO−
4 + 6 H+ → 5 NO−
3 + 2 Mn2+ + 3 H2O
The product of reduction reactions with nitrite ion are varied, depending on the reducing agent used and its strength. With sulfur dioxide, the products are NO and N2O; with tin(II) (Sn2+) the product is hyponitrous acid (H2N2O2); reduction all the way to ammonia (NH3) occurs with hydrogen sulfide. With the hydrazinium cation (N
2H+
5) the product is hydrazoic acid (HN3), an explosive compound:
- HNO2 + N
2H+
5 → HN3 + H2O + H3O+
which can also further react with nitrite:
- HNO2 + HN3 → N2O + N2 + H2O
This reaction is unusual in that it involves compounds with nitrogen in four different oxidation states.[2]
Analysis of nitrite
Nitrite is detected and analyzed by the Griess Reaction, involving the formation of a deep red-colored azo dye upon treatment of a NO−
2-containing sample with sulfanilic acid and naphthyl-1-amine in the presence of acid.[5]
Coordination complexes
Nitrite is an ambidentate ligand and can form a wide variety of coordination complexes by binding to metal ions in several ways:[2]
- When donation is from the nitrogen atom to a metal center, the complex is known as a nitro- complex.
- When donation is from one oxygen atom to a metal center, the complex is known as a nitrito- complex.
- Both oxygen atoms may donate to a metal center, forming a chelate complex.
- A nitrite ion can form an unsymmetrical bridge between two metal centers, donating through nitrogen to one metal, and through oxygen to the other.
- A single oxygen atom can bridge to two metal centers.
The red nitrito complex [Co(NH3)5(ONO)]2+ is metastable, isomerizing to the yellow nitro complex [Co(NH3)5(NO2)]2+. An example of chelating nitrite is [Cu(bipy)2(O2N)]NO3 – "bipy" is the bidentate ligand 2,2′-bypyridyl.[2]
Biochemistry

In nitrification, ammonium is converted to nitrite. Important species include Nitrosomonas. Other bacterial species such as Nitrobacter, are responsible for the oxidation of the nitrite into nitrate.
Nitrite can be reduced to nitric oxide or ammonia by many species of bacteria. Under hypoxic conditions, nitrite may release nitric oxide, which causes potent vasodilation. Several mechanisms for nitrite conversion to NO have been described, including enzymatic reduction by xanthine oxidoreductase, nitrite reductase, and NO synthase (NOS), as well as nonenzymatic acidic disproportionation reactions.
Toxicity
The existence of nitrite ions in water samples and human food product sources can cause various human diseases. For example, nitrites can produce N-nitrosamines in the presence of secondary amines which are likely to cause stomach cancer. These materials can also react with hemoglobin producing methemoglobin which decreases blood oxygen-carrying capacity in the concentration of 50 mg kg−1 of baby foods in infants and young children. It should be added that the presence of nitrate can cause the same effect due to its transformation to nitrite in the digestive system and/or by a microbial reduction in food products.[8]
Uses
Chemical precursor
Azo dyes and other colorants are prepared by the process called diazotization, which requires nitrite.[1]
Nitrite in food preservation and biochemistry
Sodium nitrite is used for the curing of meat because it prevents bacterial growth, specifically preventing botulism. Nitrite inhibits the germination of endospores of C. botulinum. In the U.S., meat cannot be labeled as "cured" without the addition of nitrite.[9][10][11] In some countries, cured-meat products are manufactured without nitrate or nitrite, and without nitrite from vegetable source.[12]
In mice, food rich in nitrites together with unsaturated fats can prevent hypertension, which is one explanation for the apparent health effect of the Mediterranean diet.[13] The recommended maximum limits by the World Health Organization in drinking water are 50 mg L−1 and 3 mg L−1 for nitrite and nitrate ions, respectively.[8]
Curing of meat
In a reaction with the meat's myoglobin, nitrite gives the product a desirable pink-red "fresh" color, such as with corned beef. This use of nitrite started the Middle Ages.[14] Historians[15] and epidemiologists[16] argue that the widespread use of nitrite in meat-curing is closely linked to the development of industrial meat-processing. In the US, nitrite has been formally used since 1925.
Organic nitrites

In organic chemistry, nitrites are esters of nitrous acid and contain the nitrosoxy functional group. Nitro compounds contain the C–NO2 group. Nitrites have the general formula RONO, where R is an aryl or alkyl group. Amyl nitrite and other alkyl nitrites are used in medicine for the treatment of heart diseases, and occasionally used recreationally for their "rush" and prolongation of orgasm, particularly in males. A classic named reaction for the synthesis of alkyl nitrites is the Meyer synthesis[17][18] in which alkyl halides react with metallic nitrites to a mixture to nitroalkanes and nitrites.
Safety
Nitrite (ingested) under conditions that result in endogenous nitrosation has been classified as "Probably carcinogenic to humans" (Group 2A) by International Agency for Research on Cancer (IARC), the specialized cancer agency of the World Health Organization (WHO) of the United Nations.[19][20]
See also
References
- ^ a b c Laue, Wolfgang; Thiemann, Michael; Scheibler, Erich; Wiegand, Karl Wilhelm (2006). "Nitrates and Nitrites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_265. ISBN 978-3527306732.
{{cite encyclopedia}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d e Greenwood, pp 461–464
- ^ IUPAC SC-Database A comprehensive database of published data on equilibrium constants of metal complexes and ligands
- ^ Greenwood, p 431
- ^ V. M. Ivanov (2004). "The 125th Anniversary of the Griess Reagent". Journal of Analytical Chemistry. 59 (10): 1002–1005. doi:10.1023/B:JANC.0000043920.77446.d7. Translated from V. M. Ivanov (2004). Zhurnal Analiticheskoi Khimii. 59 (10): 1109–1112.
{{cite journal}}: Missing or empty|title=(help) - ^ Sparacino-Watkins, Courtney; Stolz, John F.; Basu, Partha (16 December 2013). "Nitrate and periplasmic nitrate reductases". Chem. Soc. Rev. 43 (2): 676–706. doi:10.1039/c3cs60249d. ISSN 1460-4744. PMC 4080430. PMID 24141308.
- ^ Simon, Jörg; Klotz, Martin G. (2013). "Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1827 (2): 114–135. doi:10.1016/j.bbabio.2012.07.005. PMID 22842521.
- ^ a b Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. (2017). "Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate". Journal of Hazardous Materials. 324 (Pt B): 762–772. doi:10.1016/j.jhazmat.2016.11.055. PMID 27894754.
- ^ De Vries, John (1997). Food Safety and Toxicity. CRC Press. p. 70. ISBN 978-0-8493-9488-1.
- ^ sodium nitrite and nitrate facts Accessed Dec 12, 2014
- ^ Doyle, Michael P.; Sperber, William H. (23 September 2009). Compendium of the Microbiological Spoilage of Foods and Beverages. p. 78. ISBN 9781441908261.
- ^ Wilson, Bee (1 March 2018). "Yes, bacon really is killing us". The Guardian. ISSN 0261-3077. Retrieved 8 February 2019.
- ^ Charles, R. L.; Rudyk, O.; Prysyazhna, O.; Kamynina, A.; Yang, J.; Morisseau, C.; Hammock, B. D.; Freeman, B. A.; Eaton, P. (2014). "Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase". Proceedings of the National Academy of Sciences. 111 (22): 8167–72. Bibcode:2014PNAS..111.8167C. doi:10.1073/pnas.1402965111. PMC 4050620. PMID 24843165.
- ^ Binkerd, E. F; Kolari, O. E (1 January 1975). "The history and use of nitrate and nitrite in the curing of meat". Food and Cosmetics Toxicology. 13 (6): 655–661. doi:10.1016/0015-6264(75)90157-1. ISSN 0015-6264. PMID 1107192.
- ^ coudray, guillaume, eric (2017). Cochonneries : comment la charcuterie est devenue un poison. paris: Decouverte. pp. part I, chapter 2 (p. 40–55) and chapter 3 (p. 56–70). ISBN 9782707193582. OCLC 1011036745.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Lauer, Klaus (1 January 1991). "The history of nitrite in human nutrition: A contribution from German cookery books". Journal of Clinical Epidemiology. 44 (3): 261–264. doi:10.1016/0895-4356(91)90037-A. ISSN 0895-4356. PMID 1999685.
- ^ Victor Meyer (1872). "Ueber die Nitroverbindungen der Fettreihe". Justus Liebig's Annalen der Chemie. 171 (1): 1–56. doi:10.1002/jlac.18741710102.; Victor Meyer, J. Locher (1876). "Ueber die Pseudonitrole, die Isomeren der Nitrolsäuren". Justus Liebig's Annalen der Chemie. 180 (1–2): 133–55. doi:10.1002/jlac.18761800113.; V. Meyer and Stüber (1872). "Vorläufige Mittheilung". Chemische Berichte. 5: 203–05. doi:10.1002/cber.18720050165.; Victor Meyer, O. Stüber (1872). "Ueber die Nitroverbindungen der Fettreihe". Chemische Berichte. 5: 399–406. doi:10.1002/cber.187200501121.; Victor Meyer, A. Rilliet (1872). "Ueber die Nitroverbindungen der Fettreiche. Dritte Mittheilung". Chemische Berichte. 5 (2): 1029–34. doi:10.1002/cber.187200502133.; Victor Meyer, C. Chojnacki (1872). "Ueber die Nitroverbindungen der Fettreihe. Vierte Mittheilung". Chemische Berichte. 5 (2): 1034–38. doi:10.1002/cber.187200502134.
- ^ Robert B. Reynolds, Homer Adkins (1929). "The Relationship of the Constitution of Certain Alky Halides to the Formation of Nitroparaffins and Alkyl Nitrites". Journal of the American Chemical Society. 51 (1): 279–87. doi:10.1021/ja01376a037.
- ^ "List of classifications, Volumes 1–116 - IARC Monographs on the Evaluation of Carcinogenic Risks to Humans". International Agency for Research on Cancer (IARC) - World Health Organization (WHO). 2010. Archived from the original on 10 June 2017. Retrieved 25 September 2016.
- ^ VOLUME 94 - Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins - IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2010. ISBN 9789283212942. Retrieved 25 September 2016.
{{cite book}}:|work=ignored (help)
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.


