Carbon nanotube
| Part of a series of articles on |
| Nanomaterials |
|---|
 |
| Carbon nanotubes |
| Fullerenes |
| Other nanoparticles |
| Nanostructured materials |

Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical nanostructure. These cylindrical carbon molecules have unusual properties, which are valuable for nanotechnology, electronics, optics and other fields of materials science and technology. Owing to the material's exceptional strength and stiffness, nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1,[1] significantly larger than for any other material.
In addition, owing to their extraordinary thermal conductivity, mechanical, and electrical properties, carbon nanotubes find applications as additives to various structural materials. For instance, nanotubes form a tiny portion of the material(s) in some (primarily carbon fiber) baseball bats, golf clubs, car parts or damascus steel.[2][3]
Nanotubes are members of the fullerene structural family. Their name is derived from their long, hollow structure with the walls formed by one-atom-thick sheets of carbon, called graphene. These sheets are rolled at specific and discrete ("chiral") angles, and the combination of the rolling angle and radius decides the nanotube properties; for example, whether the individual nanotube shell is a metal or semiconductor. Nanotubes are categorized as single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs). Individual nanotubes naturally align themselves into "ropes" held together by van der Waals forces, more specifically, pi-stacking.
Applied quantum chemistry, specifically, orbital hybridization best describes chemical bonding in nanotubes. The chemical bonding of nanotubes is composed entirely of sp2 bonds, similar to those of graphite. These bonds, which are stronger than the sp3 bonds found in alkanes and diamond, provide nanotubes with their unique strength.
Types of carbon nanotubes and related structures
Terminology
There is no consensus on some terms describing carbon nanotubes in scientific literature: both "-wall" and "-walled" are being used in combination with "single", "double", "triple" or "multi", and the letter C is often omitted in the abbreviation; for example, multi-walled carbon nanotube (MWNT).
Single-walled
-
Armchair (n,n) i.e.: m=n
-
The translation vector is bent, while the chiral vector stays straight
-
Graphene nanoribbon
-
The chiral vector is bent, while the translation vector stays straight
-
Zigzag (n,0)
-
Chiral (n,m)
-
n and m can be counted at the end of the tube
-
Graphene nanoribbon
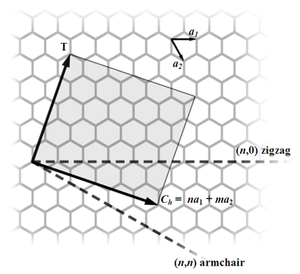


Most single-walled nanotubes (SWNTs) have a diameter of close to 1 nanometer, and can be many millions of times longer. The structure of a SWNT can be conceptualized by wrapping a one-atom-thick layer of graphite called graphene into a seamless cylinder. The way the graphene sheet is wrapped is represented by a pair of indices (n,m). The integers n and m denote the number of unit vectors along two directions in the honeycomb crystal lattice of graphene. If m = 0, the nanotubes are called zigzag nanotubes, and if n = m, the nanotubes are called armchair nanotubes. Otherwise, they are called chiral. The diameter of an ideal nanotube can be calculated from its (n,m) indices as follows
where a = 0.246 nm.
SWNTs are an important variety of carbon nanotube because most of their properties change significantly with the (n,m) values, and this dependence is non-monotonic (see Kataura plot). In particular, their band gap can vary from zero to about 2 eV and their electrical conductivity can show metallic or semiconducting behavior. Single-walled nanotubes are likely candidates for miniaturizing electronics. The most basic building block of these systems is the electric wire, and SWNTs with diameters of an order of a nanometer can be excellent conductors.[4][5] One useful application of SWNTs is in the development of the first intermolecular field-effect transistors (FET). The first intermolecular logic gate using SWCNT FETs was made in 2001.[6] A logic gate requires both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to oxygen and n-FETs otherwise, it is possible to expose half of an SWNT to oxygen and protect the other half from it. The resulting SWNT acts as a not logic gate with both p and n-type FETs in the same molecule.
Prices for single-walled nanotubes declined from around $1500 per gram as of 2000 to retail prices of around $50 per gram of as-produced 40–60% by weight SWNTs as of March 2010. As of 2016 the retail price of as-produced 75% by weight SWNTs were $2 per gram, cheap enough for widespread use.[7] SWNTs are forecast to make a large impact in electronics applications by 2020 according to the The Global Market for Carbon Nanotubes report.
Multi-walled


Multi-walled nanotubes (MWNTs) consist of multiple rolled layers (concentric tubes) of graphene. There are two models that can be used to describe the structures of multi-walled nanotubes. In the Russian Doll model, sheets of graphite are arranged in concentric cylinders, e.g., a (0,8) single-walled nanotube (SWNT) within a larger (0,17) single-walled nanotube. In the Parchment model, a single sheet of graphite is rolled in around itself, resembling a scroll of parchment or a rolled newspaper. The interlayer distance in multi-walled nanotubes is close to the distance between graphene layers in graphite, approximately 3.4 Å. The Russian Doll structure is observed more commonly. Its individual shells can be described as SWNTs, which can be metallic or semiconducting. Because of statistical probability and restrictions on the relative diameters of the individual tubes, one of the shells, and thus the whole MWNT, is usually a zero-gap metal[citation needed].
Double-walled carbon nanotubes (DWNTs) form a special class of nanotubes because their morphology and properties are similar to those of SWNTs but they are more resistant to chemicals. This is especially important when it is necessary to graft chemical functions to the surface of the nanotubes (functionalization) to add properties to the CNT. Covalent functionalization of SWNTs will break some C=C double bonds, leaving "holes" in the structure on the nanotube, and thus modifying both its mechanical and electrical properties. In the case of DWNTs, only the outer wall is modified. DWNT synthesis on the gram-scale was first proposed in 2003[8] by the CCVD technique, from the selective reduction of oxide solutions in methane and hydrogen.
The telescopic motion ability of inner shells[9] and their unique mechanical properties[10] will permit the use of multi-walled nanotubes as main movable arms in coming nanomechanical devices. Retraction force that occurs to telescopic motion caused by the Lennard-Jones interaction between shells and its value is about 1.5 nN.[11]
Torus
In theory, a nanotorus is a carbon nanotube bent into a torus (doughnut shape). Nanotori are predicted to have many unique properties, such as magnetic moments 1000 times larger than previously expected for certain specific radii.[12] Properties such as magnetic moment, thermal stability, etc. vary widely depending on radius of the torus and radius of the tube.[12][13]
Nanobud
Carbon nanobuds are a newly created material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes. In this new material, fullerene-like "buds" are covalently bonded to the outer sidewalls of the underlying carbon nanotube. This hybrid material has useful properties of both fullerenes and carbon nanotubes. In particular, they have been found to be exceptionally good field emitters.[14] In composite materials, the attached fullerene molecules may function as molecular anchors preventing slipping of the nanotubes, thus improving the composite’s mechanical properties.
Three-dimensional carbon nanotube architectures

Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>100 nm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical initiated thermal crosslinking method to fabricate macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[15] These scaffolds possess macro-, micro-, and nano- structured pores and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures may be used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis, photovoltaics, and biomedical devices and implants.[16]
Graphenated carbon nanotubes (g-CNTs)

Graphenated CNTs are a relatively new hybrid that combines graphitic foliates grown along the sidewalls of multiwalled or bamboo style CNTs. Yu et al.[17] reported on "chemically bonded graphene leaves" growing along the sidewalls of CNTs. Stoner et al.[18] described these structures as "graphenated CNTs" and reported in their use for enhanced supercapacitor performance. Hsu et al. further reported on similar structures formed on carbon fiber paper, also for use in supercapacitor applications.[19] Pham et al. [20][21] also reported a similar structure, namely "graphene-carbon nanotube hybrids", grown directly onto carbon fiber paper to form an integrated, binder free, high surface area conductive catalyst support for Proton Exchange Membrane Fuel Cells electrode applications with enhanced performance and durability. The foliate density can vary as a function of deposition conditions (e.g. temperature and time) with their structure ranging from few layers of graphene (< 10) to thicker, more graphite-like.[22]
The fundamental advantage of an integrated graphene-CNT structure is the high surface area three-dimensional framework of the CNTs coupled with the high edge density of graphene. Graphene edges provide significantly higher charge density and reactivity than the basal plane, but they are difficult to arrange in a three-dimensional, high volume-density geometry. CNTs are readily aligned in a high density geometry (i.e., a vertically aligned forest)[23] but lack high charge density surfaces—the sidewalls of the CNTs are similar to the basal plane of graphene and exhibit low charge density except where edge defects exist. Depositing a high density of graphene foliates along the length of aligned CNTs can significantly increase the total charge capacity per unit of nominal area as compared to other carbon nanostructures.[24]
Nitrogen-doped carbon nanotubes
Nitrogen doped carbon nanotubes (N-CNTs) can be produced through five main methods, chemical vapor deposition,[25][26] high-temperature and high-pressure reactions, gas-solid reaction of amorphous carbon with NH3 at high temperature,[27] solid reaction,[28] and solvothermal synthesis.[29]
N-CNTs can also be prepared by a CVD method of pyrolyzing melamine under Ar at elevated temperatures of 800–980 °C. However synthesis by CVD of melamine results in the formation of bamboo-structured CNTs. XPS spectra of grown N-CNTs reveal nitrogen in five main components, pyridinic nitrogen, pyrrolic nitrogen, quaternary nitrogen, and nitrogen oxides. Furthermore, synthesis temperature affects the type of nitrogen configuration.[26]
Nitrogen doping plays a pivotal role in lithium storage, as it creates defects in the CNT walls allowing for Li ions to diffuse into interwall space. It also increases capacity by providing more favorable bind of N-doped sites. N-CNTs are also much more reactive to metal oxide nanoparticle deposition which can further enhance storage capacity, especially in anode materials for Li-ion batteries.[30] However boron-doped nanotubes have been shown to make batteries with triple capacity.[31]
Peapod
A carbon peapod[32][33] is a novel hybrid carbon material which traps fullerene inside a carbon nanotube. It can possess interesting magnetic properties with heating and irradiation. It can also be applied as an oscillator during theoretical investigations and predictions.[34][35]
Cup-stacked carbon nanotubes
Cup-stacked carbon nanotubes (CSCNTs) differ from other quasi-1D carbon structures, which normally behave as quasi-metallic conductors of electrons. CSCNTs exhibit semiconducting behaviors due to the stacking microstructure of graphene layers.[36]
Extreme carbon nanotubes
The observation of the longest carbon nanotubes grown so far are over 1/2 m (550 mm long) was reported in 2013.[37] These nanotubes were grown on Si substrates using an improved chemical vapor deposition (CVD) method and represent electrically uniform arrays of single-walled carbon nanotubes.[1]
The shortest carbon nanotube is the organic compound cycloparaphenylene, which was synthesized in 2008.[38]
The thinnest carbon nanotube is the armchair (2,2) CNT with a diameter of 0.3 nm. This nanotube was grown inside a multi-walled carbon nanotube. Assigning of carbon nanotube type was done by a combination of high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy and density functional theory (DFT) calculations.[39]
The thinnest freestanding single-walled carbon nanotube is about 0.43 nm in diameter. Researchers suggested that it can be either (5,1) or (4,2) SWCNT, but the exact type of carbon nanotube remains questionable.[40] (3,3), (4,3) and (5,1) carbon nanotubes (all about 0.4 nm in diameter) were unambiguously identified using aberration-corrected high-resolution transmission electron microscopy inside double-walled CNTs.[41]
The highest density of CNTs was achieved in 2013, grown on a conductive titanium-coated copper surface that was coated with co-catalysts cobalt and molybdenum at lower than typical temperatures of 450 °C. The tubes averaged a height of 380 nm and a mass density of 1.6 g cm−3. The material showed ohmic conductivity (lowest resistance ∼22 kΩ).[42][43]
Properties
Strength
Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus respectively. This strength results from the covalent sp2 bonds formed between the individual carbon atoms. In 2000, a multi-walled carbon nanotube was tested to have a tensile strength of 63 gigapascals (9,100,000 psi).[44] (For illustration, this translates into the ability to endure tension of a weight equivalent to 6,422 kilograms-force (62,980 N; 14,160 lbf) on a cable with cross-section of 1 square millimetre (0.0016 sq in).) Further studies, such as one conducted in 2008, revealed that individual CNT shells have strengths of up to ≈100 gigapascals (15,000,000 psi), which is in agreement with quantum/atomistic models.[45] Since carbon nanotubes have a low density for a solid of 1.3 to 1.4 g/cm3,[46] its specific strength of up to 48,000 kN·m·kg−1 is the best of known materials, compared to high-carbon steel's 154 kN·m·kg−1.
Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% and can increase the maximum strain the tubes undergo before fracture by releasing strain energy.[citation needed]
Although the strength of individual CNT shells is extremely high, weak shear interactions between adjacent shells and tubes lead to significant reduction in the effective strength of multi-walled carbon nanotubes and carbon nanotube bundles down to only a few GPa.[47] This limitation has been recently addressed by applying high-energy electron irradiation, which crosslinks inner shells and tubes, and effectively increases the strength of these materials to ≈60 GPa for multi-walled carbon nanotubes[45] and ≈17 GPa for double-walled carbon nanotube bundles.[47]
CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional, or bending stress.[48]
| Material | Young's modulus (TPa) | Tensile strength (GPa) | Elongation at break (%) |
|---|---|---|---|
| SWNTE | ≈1 (from 1 to 5) | 13–53 | 16 |
| Armchair SWNTT | 0.94 | 126.2 | 23.1 |
| Zigzag SWNTT | 0.94 | 94.5 | 15.6–17.5 |
| Chiral SWNT | 0.92 | ||
| MWNTE | 0.2[44]–0.8[53]–0.95[44] | 11[44]–63[44]–150[53] | |
| Stainless steelE | 0.186[54]–0.214[55] | 0.38[54]–1.55[55] | 15–50 |
| Kevlar–29&149E | 0.06–0.18[56] | 3.6–3.8[56] | ≈2 |
EExperimental observation; TTheoretical prediction
The above discussion referred to axial properties of the nanotube, whereas simple geometrical considerations suggest that carbon nanotubes should be much softer in the radial direction than along the tube axis. Indeed, TEM observation of radial elasticity suggested that even the van der Waals forces can deform two adjacent nanotubes.[57] Nanoindentation experiments, performed by several groups on multiwalled carbon nanotubes[58][59] and tapping/contact mode atomic force microscope measurements performed on single-walled carbon nanotubes,[60] indicated a Young's modulus of the order of several GPa, confirming that CNTs are indeed rather soft in the radial direction.
Hardness
Standard single-walled carbon nanotubes can withstand a pressure up to 25 GPa without [plastic/permanent] deformation. They then undergo a transformation to superhard phase nanotubes. Maximum pressures measured using current experimental techniques are around 55 GPa. However, these new superhard phase nanotubes collapse at an even higher, albeit unknown, pressure.[citation needed]
The bulk modulus of superhard phase nanotubes is 462 to 546 GPa, even higher than that of diamond (420 GPa for single diamond crystal).[61]
Wettability
The surface wettability of CNT is of importance for its applications in various settings. Although the intrinsic contact angle of graphite is around 90°, the contact angles of most as-synthesized CNT arrays are over 160°, exhibiting a superhydrophobic property. By applying a voltage as low as 1.3V, the extreme water repellant surface can be switched to a superhydrophilic one.[62]
Kinetic properties
Multi-walled nanotubes are multiple concentric nanotubes precisely nested within one another. These exhibit a striking telescoping property whereby an inner nanotube core may slide, almost without friction, within its outer nanotube shell, thus creating an atomically perfect linear or rotational bearing. This is one of the first true examples of molecular nanotechnology, the precise positioning of atoms to create useful machines. Already, this property has been utilized to create the world's smallest rotational motor.[63] Future applications such as a gigahertz mechanical oscillator are also envisioned.[citation needed]
Electrical properties

Unlike graphene, which is a two-dimensional semimetal, carbon nanotubes are either metallic or semiconducting along the tubular axis. For a given (n,m) nanotube, if n = m, the nanotube is metallic; if n − m is a multiple of 3, then the nanotube is semiconducting with a very small band gap, otherwise the nanotube is a moderate semiconductor. Thus all armchair (n = m) nanotubes are metallic, and nanotubes (6,4), (9,1), etc. are semiconducting.[64] Carbon nanotubes are not semimetallic because the degenerate point (that point where the π [bonding] band meets the π* [anti-bonding] band, at which the energy goes to zero) is slightly shifted away from the K point in the Brillouin zone due to the curvature of the tube surface, casing hybridization between the σ* and π* anti-bonding bands, modifying the band dispersion.
The rule regarding metallic versus semiconductor behavior has exceptions, because curvature effects in small diameter tubes can strongly influence electrical properties. Thus, a (5,0) SWCNT that should be semiconducting in fact is metallic according to the calculations. Likewise, zigzag and chiral SWCNTs with small diameters that should be metallic have a finite gap (armchair nanotubes remain metallic).[64] In theory, metallic nanotubes can carry an electric current density of 4 × 109 A/cm2, which is more than 1,000 times greater than those of metals such as copper,[65] where for copper interconnects current densities are limited by electromigration. Carbon nanotubes are thus being explored as conductivity enhancing components in composite materials and many groups are attempting to commercialize highly conducting electrical wire assembled from individual carbon nanotubes. There are significant challenges to be overcome, however, such as the much more resistive nanotube-to-nanotube junctions and impurities, all of which lower the electrical conductivity of the macroscopic nanotube wires by orders of magnitude, as compared to the conductivity of the individual nanotubes.
Because of its nanoscale cross-section, electrons propagate only along the tube's axis. As a result, carbon nanotubes are frequently referred to as one-dimensional conductors. The maximum electrical conductance of a single-walled carbon nanotube is 2G0, where G0 = 2e2/h is the conductance of a single ballistic quantum channel.[66]
Due to the role of the π-electron system in determining the electronic properties of graphene, doping in carbon nanotubes differs from that of bulk crystalline semiconductors from the same group of the periodic table (e.g. silicon). Graphitic substitution of carbon atoms in the nanotube wall by boron or nitrogen dopants leads to p-type and n-type behavior, respectively, as would be expected in silicon. However, some non-substitutional (intercalated or adsorbed) dopants introduced into a carbon nanotube, such as alkali metals as well as electron-rich metallocenes, result in n-type conduction because they donate electrons to the π-electron system of the nanotube. By contrast, π-electron acceptors such as FeCl3 or electron-deficient metallocenes function as p-type dopants since they draw π-electrons away from the top of the valence band.
Intrinsic superconductivity has been reported,[67] although other experiments found no evidence of this, leaving the claim a subject of debate.[68]
Optical properties
Thermal properties
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction", but good insulators lateral to the tube axis. Measurements show that a SWNT has a room-temperature thermal conductivity along its axis of about 3500 W·m−1·K−1;[69] compare this to copper, a metal well known for its good thermal conductivity, which transmits 385 W·m−1·K−1. A SWNT has a room-temperature thermal conductivity across its axis (in the radial direction) of about 1.52 W·m−1·K−1,[70] which is about as thermally conductive as soil. The temperature stability of carbon nanotubes is estimated to be up to 2800 °C in vacuum and about 750 °C in air.[71]
Defects
As with any material, the existence of a crystallographic defect affects the material properties. Defects can occur in the form of atomic vacancies. High levels of such defects can lower the tensile strength by up to 85%. An important example is the Stone Wales defect, which creates a pentagon and heptagon pair by rearrangement of the bonds. Because of the very small structure of CNTs, the tensile strength of the tube is dependent on its weakest segment in a similar manner to a chain, where the strength of the weakest link becomes the maximum strength of the chain.
Crystallographic defects also affect the tube's electrical properties. A common result is lowered conductivity through the defective region of the tube. A defect in armchair-type tubes (which can conduct electricity) can cause the surrounding region to become semiconducting, and single monatomic vacancies induce magnetic properties.[72]
Crystallographic defects strongly affect the tube's thermal properties. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path and reduces the thermal conductivity of nanotube structures. Phonon transport simulations indicate that substitutional defects such as nitrogen or boron will primarily lead to scattering of high-frequency optical phonons. However, larger-scale defects such as Stone Wales defects cause phonon scattering over a wide range of frequencies, leading to a greater reduction in thermal conductivity.[73]
Safety and health
The toxicity of carbon nanotubes has been an important question in nanotechnology. As of 2007, such research had just begun. The data is still fragmentary and subject to criticism. Preliminary results highlight the difficulties in evaluating the toxicity of this heterogeneous material. Parameters such as structure, size distribution, surface area, surface chemistry, surface charge, and agglomeration state as well as purity of the samples, have considerable impact on the reactivity of carbon nanotubes. However, available data clearly show that, under some conditions, nanotubes can cross membrane barriers, which suggests that, if raw materials reach the organs, they can induce harmful effects such as inflammatory and fibrotic reactions.[74][75]
As of October 2016, single wall carbon nanotubes have been registered through the European Union's Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulations, based on evaluation of the potentially hazardous properties of SWCNT. Based on this registration, SWCNT commercialization is allowed in the EU up to 10 metric tons. Currently, the type of SWCNT registered through REACH is limited to the specific type of single wall carbon nanotubes manufactured by OCSiAl, which submitted the application.[76]
Synthesis
Techniques have been developed to produce nanotubes in sizable quantities, including arc discharge, laser ablation, high-pressure carbon monoxide disproportionation, and chemical vapor deposition (CVD). Most of these processes take place in a vacuum or with process gases. CVD growth of CNTs can occur in vacuum or at atmospheric pressure. Large quantities of nanotubes can be synthesized by these methods; advances in catalysis and continuous growth are making CNTs more commercially viable.[77]
Chemical modification
Carbon nanotubes can be functionalized to attain desired properties that can be used in a wide variety of applications. The two main methods of carbon nanotube functionalization are covalent and non-covalent modifications. Because of their hydrophobic nature, carbon nanotubes tend to agglomerate hindering their dispersion is solvents or viscous polymer melts. The resulting nanotube bundles or aggregates reduce the mechanical performance of the final composite. The surface of the carbon nanotubes can be modified to reduce the hydrophobicity and improve interfacial adhesion to a bulk polymer through chemical attachment.[78]
Applications
Current
Current use and application of nanotubes has mostly been limited to the use of bulk nanotubes, which is a mass of rather unorganized fragments of nanotubes. Bulk nanotube materials may never achieve a tensile strength similar to that of individual tubes, but such composites may, nevertheless, yield strengths sufficient for many applications. Bulk carbon nanotubes have already been used as composite fibers in polymers to improve the mechanical, thermal and electrical properties of the bulk product.
- Easton-Bell Sports, Inc. have been in partnership with Zyvex Performance Materials, using CNT technology in a number of their bicycle components—including flat and riser handlebars, cranks, forks, seatposts, stems and aero bars.
- Zyvex Technologies has also built a 54' maritime vessel, the Piranha Unmanned Surface Vessel, as a technology demonstrator for what is possible using CNT technology. CNTs help improve the structural performance of the vessel, resulting in a lightweight 8,000 lb boat that can carry a payload of 15,000 lb over a range of 2,500 miles.[79]
- Amroy Europe Oy manufactures Hybtonite carbon nanoepoxy resins where carbon nanotubes have been chemically activated to bond to epoxy, resulting in a composite material that is 20% to 30% stronger than other composite materials. It has been used for wind turbines, marine paints and variety of sports gear such as skis, ice hockey sticks, baseball bats, hunting arrows, and surfboards.[80]
Other current applications include:
- tips for atomic force microscope probes[81]
- in tissue engineering, carbon nanotubes can act as scaffolding for bone growth[82]
There is also ongoing research in using carbon nanotubes as a scaffold for diverse microfabrication techniques.[83]
Potential
The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering. The highest tensile strength of an individual multi-walled carbon nanotube has been tested to be 63 GPa.[44] Carbon nanotubes were found in Damascus steel from the 17th century, possibly helping to account for the legendary strength of the swords made of it.[84][85] Recently, several studies have highlighted the prospect of using carbon nanotubes as building blocks to fabricate three-dimensional macroscopic (>1mm in all three dimensions) all-carbon devices. Lalwani et al. have reported a novel radical initiated thermal crosslinking method to fabricated macroscopic, free-standing, porous, all-carbon scaffolds using single- and multi-walled carbon nanotubes as building blocks.[15] These scaffolds possess macro-, micro-, and nano- structured pores and the porosity can be tailored for specific applications. These 3D all-carbon scaffolds/architectures maybe used for the fabrication of the next generation of energy storage, supercapacitors, field emission transistors, high-performance catalysis, photovoltaics, and biomedical devices and implants.
Discovery
The true identity of the discoverers of carbon nanotubes is a subject of some controversy.[86] For years, scientists assumed that Sumio Iijima of NEC had discovered carbon nanotubes in 1991. He published a paper describing his discovery which initiated a flurry of excitement and could be credited by inspiring the many scientists now studying applications of carbon nanotubes. Though Iijima has been given much of the credit for discovering carbon nanotubes, it turns out that the timeline of carbon nanotubes goes back much further than 1991.[86] In 1952 L. V. Radushkevich and V. M. Lukyanovich published clear images of 50 nanometer diameter tubes made of carbon in the Soviet Journal of Physical Chemistry.[87] This discovery was largely unnoticed, as the article was published in Russian, and Western scientists' access to Soviet press was limited during the Cold War. Before they came to be known as carbon nanotubes, in 1976, Morinobu Endo of CNRS observed hollow tubes of rolled up graphite sheets synthesised by a chemical vapour-growth technique.[88] The first specimens observed would later come to be known as single-walled carbon nanotubes (SWNTs).[89] The three scientists have been the first ones to show images of a nanotube with a solitary graphene wall.[86]
Endo, in his early review of vapor-phase-grown carbon fibers (VPCF), also reminded us that he had observed a hollow tube, linearly extended with parallel carbon layer faces near the fiber core.[90] This appears to be the observation of multi-walled carbon nanotubes at the center of the fiber.[89] The mass-produced MWCNTs today are strongly related to the VPGCF developed by Endo.[89] In fact, they call it the “Endo-process”, out of respect for his early work and patents.[89][91]
In 1979, John Abrahamson presented evidence of carbon nanotubes at the 14th Biennial Conference of Carbon at Pennsylvania State University. The conference paper described carbon nanotubes as carbon fibers that were produced on carbon anodes during arc discharge. A characterization of these fibers was given as well as hypotheses for their growth in a nitrogen atmosphere at low pressures.[92]
In 1981, a group of Soviet scientists published the results of chemical and structural characterization of carbon nanoparticles produced by a thermocatalytical disproportionation of carbon monoxide. Using TEM images and XRD patterns, the authors suggested that their “carbon multi-layer tubular crystals” were formed by rolling graphene layers into cylinders. They speculated that by rolling graphene layers into a cylinder, many different arrangements of graphene hexagonal nets are possible. They suggested two possibilities of such arrangements: circular arrangement (armchair nanotube) and a spiral, helical arrangement (chiral tube).[93]
In 1987, Howard G. Tennent of Hyperion Catalysis was issued a U.S. patent for the production of "cylindrical discrete carbon fibrils" with a "constant diameter between about 3.5 and about 70 nanometers..., length 102 times the diameter, and an outer region of multiple essentially continuous layers of ordered carbon atoms and a distinct inner core...."[94]
Iijima's discovery of multi-walled carbon nanotubes in the insoluble material of arc-burned graphite rods in 1991[95] and Mintmire, Dunlap, and White's independent prediction that if single-walled carbon nanotubes could be made, then they would exhibit remarkable conducting properties[96] helped create the initial buzz that is now associated with carbon nanotubes. Nanotube research accelerated greatly following the independent discoveries[97][98] by Bethune at IBM and Iijima at NEC of single-walled carbon nanotubes and methods to specifically produce them by adding transition-metal catalysts to the carbon in an arc discharge. The arc discharge technique was well-known to produce the famed Buckminster fullerene on a preparative scale,[99] and these results appeared to extend the run of accidental discoveries relating to fullerenes. The discovery of nanotubes remains a contentious issue. Many believe that Iijima's report in 1991 is of particular importance because it brought carbon nanotubes into the awareness of the scientific community as a whole.[86][89]
See also
- Boron nitride nanotube
- Buckypaper
- Carbide-derived carbon
- Carbon nanocone
- Carbon nanofibers
- Carbon nanoparticles
- Carbon nanoscrolls
- Carbon nanotube chemistry
- Colossal carbon tube
- Diamond nanothread
- Filamentous carbon
- Graphene oxide paper
- List of software for nanostructures modeling
- Molecular modelling
- Nanoflower
- Ninithi (nanotube modelling software)
- Organic semiconductor
- Selective chemistry of single-walled nanotubes
- Silicon nanotubes
- Timeline of carbon nanotubes
- Vantablack, a substance produced in 2014; the blackest substance known
References
This article incorporates public domain text from National Institute of Environmental Health Sciences (NIEHS) as quoted.
- ^ a b Wang, X.; Li, Qunqing; Xie, Jing; Jin, Zhong; Wang, Jinyong; Li, Yan; Jiang, Kaili; Fan, Shoushan (2009). "Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates". Nano Letters. 9 (9): 3137–3141. Bibcode:2009NanoL...9.3137W. doi:10.1021/nl901260b. PMID 19650638.
- ^ Legendary Swords' Sharpness, Strength From Nanotubes, Study Says
- ^ Gullapalli, S.; Wong, M.S. (2011). "Nanotechnology: A Guide to Nano-Objects" (PDF). Chemical Engineering Progress. 107 (5): 28–32.
- ^ Mintmire, J.W.; Dunlap, B.I.; White, C.T. (1992). "Are Fullerene Tubules Metallic?". Phys. Rev. Lett. 68 (5): 631–634. Bibcode:1992PhRvL..68..631M. doi:10.1103/PhysRevLett.68.631. PMID 10045950.
- ^ Dekker, C. (1999). "Carbon nanotubes as molecular quantum wires". Physics Today. 52 (5): 22–28. Bibcode:1999PhT....52e..22D. doi:10.1063/1.882658.
- ^ Martel, R.; Derycke, V.; Lavoie, C.; Appenzeller, J.; Chan, K.; Tersoff, J.; Avouris, Ph. (2001). "Ambipolar Electrical Transport in Semiconducting Single-Wall Carbon Nanotubes". Phys. Rev. Lett. 87 (25): 256805. Bibcode:2001PhRvL..87y6805M. doi:10.1103/PhysRevLett.87.256805. PMID 11736597.
- ^ Single wall carbon nanotubes at OCSiAl web site
- ^ Flahaut, E.; Bacsa, Revathi; Peigney, Alain; Laurent, Christophe (2003). "Gram-Scale CCVD Synthesis of Double-Walled Carbon Nanotubes". Chemical Communications. 12 (12): 1442–1443. doi:10.1039/b301514a. PMID 12841282.
- ^ Cumings, J.; Zettl, A. (2000). "Low-Friction Nanoscale Linear Bearing Realized from Multiwall Carbon Nanotubes". Science. 289 (5479): 602–604. Bibcode:2000Sci...289..602C. doi:10.1126/science.289.5479.602. PMID 10915618.
- ^ Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. (1996). "Exceptionally high Young's modulus observed for individual carbon nanotubes". Nature. 381 (6584): 678–680. Bibcode:1996Natur.381..678T. doi:10.1038/381678a0.
- ^ Zavalniuk, V.; Marchenko, S. (2011). "Theoretical analysis of telescopic oscillations in multi-walled carbon nanotubes". Low Temperature Physics. 37 (4): 337. arXiv:0903.2461. Bibcode:2011LTP....37..337Z. doi:10.1063/1.3592692.
- ^ a b Liu, L.; Guo, G.; Jayanthi, C.; Wu, S. (2002). "Colossal Paramagnetic Moments in Metallic Carbon Nanotori". Phys. Rev. Lett. 88 (21): 217206. Bibcode:2002PhRvL..88u7206L. doi:10.1103/PhysRevLett.88.217206. PMID 12059501.
- ^ Huhtala, M.; Kuronen, A.; Kaski, K. (2002). "Carbon nanotube structures: Molecular dynamics simulation at realistic limit" (PDF). Computer Physics Communications. 146 (1): 30–37. Bibcode:2002CoPhC.146...30H. doi:10.1016/S0010-4655(02)00432-0. Archived from the original (PDF) on 27 June 2008.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Nasibulin, Albert G.; et al. (2007). "A novel hybrid carbon material" (PDF). Nature Nanotechnology. 2 (3): 156–161. doi:10.1038/nnano.2007.37. PMID 18654245.
- ^ a b Balaji Sitharaman., Lalwani, Gaurav, Andrea Trinward Kwaczala, Shruti Kanakia, Sunny C. Patel, Stefan Judex (2013). "Fabrication and characterization of three-dimensional macroscopic all-carbon scaffolds". Carbon. 53: 90–100. doi:10.1016/j.carbon.2012.10.035. PMC 3578711. PMID 23436939.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Balaji Sitharaman., Lalwani, Gaurav, Anu Gopalan, Michael D'Agati, Jeyantt Srinivas Sankaran, Stefan Judex, Yi-Xian Qin, (2015). "Porous three-dimensional carbon nanotube scaffolds for tissue engineering". Journal of Biomedical Materials Research Part A. 103: 3212–3225. doi:10.1002/jbm.a.35449. PMID 25788440.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Yu, Kehan; Ganhua Lu; Zheng Bo; Shun Mao; Junhong Chen (2011). "Carbon Nanotube with Chemically Bonded Graphene Leaves for Electronic and Optoelectronic Applications". J. Phys. Chem. Lett. 13. 2 (13): 1556–1562. doi:10.1021/jz200641c.
- ^ Stoner, Brian R.; Akshay S. Raut; Billyde Brown; Charles B. Parker; Jeffrey T. Glass (2011). "Graphenated carbon nanotubes for enhanced electrochemical double layer capacitor performance". Appl. Phys. Lett. 18. 99 (18): 183104. Bibcode:2011ApPhL..99r3104S. doi:10.1063/1.3657514.
- ^ Hsu, Hsin-Cheng; Wang, Chen-Hao; Nataraj, S.K.; Huang, Hsin-Chih; Du, He-Yun; Chang, Sun-Tang; Chen, Li-Chyong; Chen, Kuei-Hsien (2012). "Stand-up structure of graphene-like carbon nanowalls on CNT directly grown on polyacrylonitrile-based carbon fiber paper as supercapacitor". Diamond and Related Materials. 25: 176–9. Bibcode:2012DRM....25..176H. doi:10.1016/j.diamond.2012.02.020.
- ^ Pham, Kien-Cuong; Chua, Daniel H.C.; McPhail, David S.; Wee, Andrew T.S. (2014). "The Direct Growth of Graphene-Carbon Nanotube Hybrids as Catalyst Support for High-Performance PEM Fuel Cells". ECS Electrochemistry Letters. 3: F37–F40. doi:10.1149/2.009406eel.
- ^ Pham, Kien-Cuong; McPhail, David S.; Mattevi, Cecilia; Wee, Andrew T.S.; Chua, Daniel H. C. (2016). "Graphene-Carbon Nanotube Hybrids as Robust Catalyst Supports in Proton Exchange Membrane Fuel Cells". Journal of The Electrochemical Society. 163: F255–F263. doi:10.1149/2.0891603jes.
- ^ Parker, Charles B.; Akshay S. Raut; Billyde Brown; Brian R. Stoner; Jeffrey T. Glass (2012). "Three-dimensional arrays of graphenated carbon nanotubes". J. Mater. Res. 7. 27 (7): 1046–53. Bibcode:2012JMatR..27.1046P. doi:10.1557/jmr.2012.43.
- ^ Cui, Hong-tao; O. Zhou; B. R. Stoner (2000). "Deposition of aligned bamboo-like carbon nanotubes via microwave plasma enhanced chemical vapor deposition". J. Appl. Phys. 88 (10): 6072–4. Bibcode:2000JAP....88.6072C. doi:10.1063/1.1320024.
- ^ Stoner, Brian R.; Jeffrey T. Glass (2012). "Carbon nanostructures: a morphological classification for charge density optimization". Diamond and Related Materials. 23: 130–4. Bibcode:2012DRM....23..130S. doi:10.1016/j.diamond.2012.01.034.
- ^ Kouvetakis, J.; Todd, M.; Wilkens, B.; Bandari, A.; Cave, N. (1994). "Novel Synthetic Routes to Carbon-Nitrogen Thin Films". Chemistry of Materials. 6 (6): 811–814. doi:10.1021/cm00042a018.
- ^ a b Zhong, Y.; Jaidann, M.; Zhang, Y.; Zhang, G.; Liu, H.; Ioan Ionescu, M.; Li, R.; Sun, X.; Abou-Rachid, H.; Lussier, L. S. (2010). "Synthesis of high nitrogen doping of carbon nanotubes and modeling the stabilization of filled DAATO@CNTs (10,10) for nanoenergetic materials". Journal of Physics and Chemistry of Solids. 71 (2): 134–139. Bibcode:2010JPCS...71..134Z. doi:10.1016/j.jpcs.2009.07.030.
- ^ Yin, L. -W.; Bando, Y.; Li, M. -S.; Liu, Y. -X.; Qi, Y. -X. (2003). "Unique Single-Crystalline Beta Carbon Nitride Nanorods". Advanced Materials. 15 (21): 1840–1844. doi:10.1002/adma.200305307.
- ^ Oku, T.; Kawaguchi, M. (2000). "Microstructure analysis of CN-based nanocage materials by high-resolution electron microscopy". Diamond and Related Materials. 9 (3–6): 906–910. Bibcode:2000DRM.....9..906O. doi:10.1016/S0925-9635(99)00359-3.
- ^ Guo, Q.; Xie, Y.; Wang, X.; Zhang, S.; Hou, T.; Lv, S. (2004). "Synthesis of carbon nitride nanotubes with the C3N4 stoichiometry via a benzene-thermal process at low temperatures Electronic Supplementary Information (ESI) available: XRD patterns". Chemical Communications: 26. doi:10.1039/B311390F.
- ^ Shin, W. H.; Jeong, H. M.; Kim, B. G.; Kang, J. K.; Choi, J. W. (2012). "Nitrogen-Doped Multiwall Carbon Nanotubes for Lithium Storage with Extremely High Capacity". Nano Letters. 12 (5): 2283–8. Bibcode:2012NanoL..12.2283S. doi:10.1021/nl3000908. PMID 22452675.
- ^ "Doped nanotubes boost lithium battery power three-fold." The Register. 14 February 2013.
- ^ Smith, Brian W.; Monthioux, Marc; Luzzi, David E. (1998). "Encapsulated C-60 in carbon nanotubes". Nature. 396 (6709): 323–324. Bibcode:1998Natur.396R.323S. doi:10.1038/24521.
- ^ Smith, B.W.; Luzzi, D.E. (2000). "Formation mechanism of fullerene peapods and coaxial tubes: a path to large scale synthesis". Chem. Phys. Lett. 321 (1–2): 169–174. Bibcode:2000CPL...321..169S. doi:10.1016/S0009-2614(00)00307-9.
- ^ Su, H.; Goddard, W.A.; Zhao, Y. (2006). "Dynamic friction force in a carbon peapod oscillator". Nanotechnology. 17 (22): 5691–5695. arXiv:cond-mat/0611671. Bibcode:2006Nanot..17.5691S. doi:10.1088/0957-4484/17/22/026.
- ^ Wang, M.; Li, C.M. (2010). "An oscillator in a carbon peapod controllable by an external electric field: A molecular dynamics study". Nanotechnology. 21 (3): 035704. Bibcode:2010Nanot..21c5704W. doi:10.1088/0957-4484/21/3/035704.
- ^ Liu, Q.; Ren, Wencai; Chen, Zhi-Gang; Yin, Lichang; Li, Feng; Cong, Hongtao; Cheng, Hui-Ming (2009). "Semiconducting properties of cup-stacked carbon nanotubes" (PDF). Carbon. 47 (3): 731–736. doi:10.1016/j.carbon.2008.11.005. Archived from the original (PDF) on 9 January 2015.
- ^ Zhang, R.; Zhang, Y.; Zhang, Q.; Xie, H.; Qian, W.; Wei, F. (2013). "Growth of Half-Meter Long Carbon Nanotubes Based on Schulz–Flory Distribution". ACS Nano. 7 (7): 6156–61. doi:10.1021/nn401995z. PMID 23806050.
- ^ "Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures". J. Am. Chem. Soc. 4 December 2008.
- ^ Zhao, X.; Liu, Y.; Inoue, S.; Suzuki, T.; Jones, R.; Ando, Y. (2004). "Smallest Carbon Nanotube is 3 Å in Diameter". Phys. Rev. Lett. 92 (12): 125502. Bibcode:2004PhRvL..92l5502Z. doi:10.1103/PhysRevLett.92.125502. PMID 15089683.
- ^ Hayashi, Takuya; Kim, Yoong Ahm; Matoba, Toshiharu; Esaka, Masaya; Nishimura, Kunio; Tsukada, Takayuki; Endo, Morinobu; Dresselhaus, Mildred S. (2003). "Smallest Freestanding Single-Walled Carbon Nanotube". Nano Letters. 3 (7): 887–889. Bibcode:2003NanoL...3..887H. doi:10.1021/nl034080r.
- ^ Guan, L.; Suenaga, K.; Iijima, S. (2008). "Smallest Carbon Nanotube Assigned with Atomic Resolution Accuracy". Nano Letters. 8 (2): 459–462. Bibcode:2008NanoL...8..459G. doi:10.1021/nl072396j. PMID 18186659.
- ^ "Densest array of carbon nanotubes grown to date". KurzweilAI. 27 September 2013.
- ^ Sugime, H.; Esconjauregui, S.; Yang, J.; d'Arsié, L.; Oliver, R. A.; Bhardwaj, S.; Cepek, C.; Robertson, J. (2013). "Low temperature growth of ultra-high mass density carbon nanotube forests on conductive supports". Applied Physics Letters. 103 (7): 073116. Bibcode:2013ApPhL.103g3116S. doi:10.1063/1.4818619.
- ^ a b c d e f Yu, M.-F.; Lourie, O; Dyer, MJ; Moloni, K; Kelly, TF; Ruoff, RS (2000). "Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load". Science. 287 (5453): 637–640. Bibcode:2000Sci...287..637Y. doi:10.1126/science.287.5453.637. PMID 10649994.
- ^ a b Peng, B.; Locascio, Mark; Zapol, Peter; Li, Shuyou; Mielke, Steven L.; Schatz, George C.; Espinosa, Horacio D. (2008). "Measurements of near-ultimate strength for multiwalled carbon nanotubes and irradiation-induced crosslinking improvements". Nature Nanotechnology. 3 (10): 626–631. doi:10.1038/nnano.2008.211. PMID 18839003.
- ^ Collins, P.G. (2000). "Nanotubes for Electronics". Scientific American: 67–69.
- ^ a b Filleter, T.; Bernal, R.; Li, S.; Espinosa, H.D. (2011). "Ultrahigh Strength and Stiffness in Cross-Linked Hierarchical Carbon Nanotube Bundles". Advanced Materials. 23 (25): 2855–2860. doi:10.1002/adma.201100547.
- ^ Jensen, K.; Mickelson, W.; Kis, A.; Zettl, A. (2007). "Buckling and kinking force measurements on individual multiwalled carbon nanotubes". Physical Review B. 76 (19): 195436. Bibcode:2007PhRvB..76s5436J. doi:10.1103/PhysRevB.76.195436.
- ^ Bellucci, S. (2005). "Carbon nanotubes: Physics and applications". Physica Status Solidi (c). 2 (1): 34–47. Bibcode:2005PSSCR...2...34B. doi:10.1002/pssc.200460105.
- ^ Chae, H.G.; Kumar, S. (2006). "Rigid Rod Polymeric Fibers". Journal of Applied Polymer Science. 100 (1): 791–802. doi:10.1002/app.22680.
- ^ Meo, M.; Rossi, M. (2006). "Prediction of Young's modulus of single wall carbon nanotubes by molecular-mechanics-based finite element modelling". Composites Science and Technology. 66 (11–12): 1597–1605. doi:10.1016/j.compscitech.2005.11.015.
- ^ Sinnott, S.B.; Andrews, R. (2001). "Carbon Nanotubes: Synthesis, Properties, and Applications". Critical Reviews in Solid State and Materials Sciences. 26 (3): 145–249. Bibcode:2001CRSSM..26..145S. doi:10.1080/20014091104189.
- ^ a b Demczyk, B.G.; Wang, Y.M; Cumings, J; Hetman, M; Han, W; Zettl, A; Ritchie, R.O (2002). "Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes". Materials Science and Engineering A. 334 (1–2): 173–178. doi:10.1016/S0921-5093(01)01807-X.
- ^ a b "Properties of Stainless Steel". Australian Stainless Steel Development Association.
- ^ a b "Stainless Steel – 17-7PH (Fe/Cr17/Ni 7) Material Information". Archived from the original on 19 July 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Wagner, H.D. (2002). "Reinforcement". Encyclopedia of Polymer Science and Technology. John Wiley & Sons. doi:10.1002/0471440264.pst317.
- ^ Ruoff, R. S.; Tersoff, J.; Lorents, D. C.; Subramoney, S.; Chan, B. (1993). "Radial deformation of carbon nanotubes by van der Waals forces". Nature. 364 (6437): 514–516. Bibcode:1993Natur.364..514R. doi:10.1038/364514a0.
- ^ Palaci, I.; Fedrigo, S.; Brune, H.; Klinke, C.; Chen, M.; Riedo, E. (2005). "Radial Elasticity of Multiwalled Carbon Nanotubes". Physical Review Letters. 94 (17). arXiv:1201.5501. Bibcode:2005PhRvL..94q5502P. doi:10.1103/PhysRevLett.94.175502.
- ^ Yu, M. F.; Kowalewski, T.; Ruoff, R. (2000). "Investigation of the Radial Deformability of Individual Carbon Nanotubes under Controlled Indentation Force". Physical Review Letters. 85 (7): 1456–9. Bibcode:2000PhRvL..85.1456Y. doi:10.1103/PhysRevLett.85.1456. PMID 10970528.
- ^ Yang, Y. H.; Li, W. Z. (2011). "Radial elasticity of single-walled carbon nanotube measured by atomic force microscopy". Applied Physics Letters. 98 (4): 041901. Bibcode:2011ApPhL..98d1901Y. doi:10.1063/1.3546170.
- ^ Popov, M.; Kyotani, M.; Nemanich, R.; Koga, Y. (2002). "Superhard phase composed of single-wall carbon nanotubes" (PDF). Phys. Rev. B. 65 (3): 033408. Bibcode:2002PhRvB..65c3408P. doi:10.1103/PhysRevB.65.033408. Archived from the original (PDF) on 20 July 2011.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Wang, Zuankai; Ci, Lijie; Chen, Li; Nayak, Saroj; Ajayan, Pulickel M.; Koratkar, Nikhil (2007). "Polarity-dependent electrochemically controlled transport of water through carbon nanotube membranes". Nano Letters. 7: 697–702. Bibcode:2007NanoL...7..697W. doi:10.1021/nl062853g. PMID 17295548.
- ^ Sanders, R. (23 March 2003). "Physicists build world's smallest motor using nanotubes and etched silicon" (Press release). UC Berkeley.
- ^ a b Lu, X.; Chen, Z. (2005). "Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (C60) and Single-Walled Carbon Nanotubes". Chemical Reviews. 105 (10): 3643–3696. doi:10.1021/cr030093d. PMID 16218563.
- ^ Hong, Seunghun; Myung, S (2007). "Nanotube Electronics: A flexible approach to mobility". Nature Nanotechnology. 2 (4): 207–208. Bibcode:2007NatNa...2..207H. doi:10.1038/nnano.2007.89. PMID 18654263.
- ^ Charlier, J. C.; Roche, S. (2007). "Electronic and transport properties of nanotubes". Reviews of Modern Physics. 79 (2): 677–732. Bibcode:2007RvMP...79..677C. doi:10.1103/RevModPhys.79.677.
- ^ Tang, Z. K.; Zhang, L; Wang, N; Zhang, XX; Wen, GH; Li, GD; Wang, JN; Chan, CT; Sheng, P (2001). "Superconductivity in 4 Angstrom Single-Walled Carbon Nanotubes". Science. 292 (5526): 2462–5. Bibcode:2001Sci...292.2462T. doi:10.1126/science.1060470. PMID 11431560.
Takesue, I.; Haruyama, J.; Kobayashi, N.; Chiashi, S.; Maruyama, S.; Sugai, T.; Shinohara, H. (2006). "Superconductivity in Entirely End-Bonded Multiwalled Carbon Nanotubes" (PDF). Phys. Rev. Lett. 96 (5): 057001. arXiv:cond-mat/0509466. Bibcode:2006PhRvL..96e7001T. doi:10.1103/PhysRevLett.96.057001. PMID 16486971.
Lortz, R.; Zhang, Q; Shi, W; Ye, J. T.; Qiu, C. Y.; Wang, Z.; He, H. T.; Sheng, P; Qian, T. Z.; Tang, Z. K.; Wang, N.; Zhang, X. X.; Wang, J; Chan, C. T. (2009). "Superconducting characteristics of 4-A carbon nanotube–zeolite composite". Proceedings of the National Academy of Sciences. 106 (18): 7299–7303. Bibcode:2009PNAS..106.7299L. doi:10.1073/pnas.0813162106. - ^ M. Bockrath (2006). "Carbon nanotubes: The weakest link". Nature Physics. 2 (3): 155–156. Bibcode:2006NatPh...2..155B. doi:10.1038/nphys252.
- ^ Pop, Eric; Mann, David; Wang, Qian; Goodson, Kenneth; Dai, Hongjie (22 December 2005). "Thermal conductance of an individual single-wall carbon nanotube above room temperature". Nano Letters. 6 (1): 96–100. arXiv:cond-mat/0512624. Bibcode:2006NanoL...6...96P. doi:10.1021/nl052145f. PMID 16402794.
- ^ Sinha, Saion; Barjami, Saimir; Iannacchione, Germano; Schwab, Alexander; Muench, George (5 June 2005). "Off-axis thermal properties of carbon nanotube films". Journal of Nanoparticle Research. 7 (6): 651–657. doi:10.1007/s11051-005-8382-9.
- ^ Thostenson, Erik; Li, C; Chou, T (2005). "Nanocomposites in context". Composites Science and Technology. 65 (3–4): 491–516. doi:10.1016/j.compscitech.2004.11.003.
- ^ Carbon-Based Magnetism: An Overview of the Magnetism of Metal Free Carbon-based Compounds and Materials, Tatiana Makarova and Fernando Palacio (eds.), Elsevier, 2006
- ^ Mingo, N.; Stewart, D. A.; Broido, D. A.; Srivastava, D. (2008). "Phonon transmission through defects in carbon nanotubes from first principles". Phys. Rev. B. 77 (3): 033418. Bibcode:2008PhRvB..77c3418M. doi:10.1103/PhysRevB.77.033418.
- ^ "Toxicity studies of carbon nanotubes". Adv Exp Med Biol. Advances in Experimental Medicine and Biology. 620: 181–204. 2007. doi:10.1007/978-0-387-76713-0_14. ISBN 978-0-387-76712-3. PMID 18217344.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Corredor, C.; Hou, W.C.; Klein, S.A.; Moghadam, B.Y.; Goryll, M.; Doudrick, K.; Westerhoff, P.; Posner, J.D. (2013). "Disruption of model cell membranes by carbon nanotubes". Carbon. 60: 67–75. doi:10.1016/j.carbon.2013.03.057.
- ^ "REACH Registration Completed for Single-Wall Carbon Nanotubes". pcimag.com. PCI Mag. 16 October 2016. Retrieved 24 November 2016.
- ^ K. Takeuchi, T. Hayashi, Y. A. Kim, K. Fujisawa, M. Endo "The state-of-the-art science and applications of carbon nanotubes", February 2014, Volume 5, Issue 1, pp 15
- ^ Karousis, Nikolaos; Tagmatarchis, Nikos; Tasis, Dimitrios (14 June 2010). "Current Progress on the Chemical Modification of Carbon Nanotubes". Chemical Reviews. 110 (9): 5366–5397. doi:10.1021/cr100018g. PMID 20545303.
- ^ "Pirahna USV built using nano-enhanced carbon prepreg". ReinforcedPlastics.com. 19 February 2009. Archived from the original on 3 March 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Pagni, John (5 March 2010). "Amroy aims to become nano-leader". European Plastics News. Archived from the original on 10 July 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Nanotube Tips". nanoScience instruments. Archived from the original on 27 October 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Haddon, Robert C.; Laura P. Zanello; Bin Zhao; Hui Hu (2006). "Bone Cell Proliferation on Carbon Nanotubes". Nano Letters. 6 (3): 562–567. Bibcode:2006NanoL...6..562Z. doi:10.1021/nl051861e. PMID 16522063.
- ^ "Publications on carbon nanotube applications including scaffold microfabrication". nano.byu.edu. 27 May 2014.
- ^ K. Sanderson (2006). "Sharpest cut from nanotube sword". Nature News. doi:10.1038/news061113-11.
- ^ Reibold, M.; Paufler, P; Levin, AA; Kochmann, W; Pätzke, N; Meyer, DC (16 November 2006). "Materials: Carbon nanotubes in an ancient Damascus sabre". Nature. 444 (7117): 286. Bibcode:2006Natur.444..286R. doi:10.1038/444286a. PMID 17108950.
- ^ a b c d Pacios Pujadó, Mercè (2012). Carbon Nanotubes as Platforms for Biosensors with Electrochemical and Electronic Transduction. Springer Heidelberg. pp. XX, 208. doi:10.1007/978-3-642-31421-6. ISBN 978-3-642-31421-6.
- ^ Радушкевич, Л. В. (1952). О Структуре Углерода, Образующегося При Термическом Разложении Окиси Углерода На Железном Контакте (PDF). Журнал Физической Химии (in Russian). 26: 88–95.
- ^ Oberlin, A.; Endo, M.; Koyama, T. (1976). "Filamentous growth of carbon through benzene decomposition". Journal of Crystal Growth. 32 (3): 335–349. Bibcode:1976JCrGr..32..335O. doi:10.1016/0022-0248(76)90115-9.
- ^ a b c d e Peter C. Eklund(Panel Chair) (2007). WTEC Panel Report on "INTERNATIONAL ASSESSMENT OF RESEARCH AND DEVELOPMENT OF CARBON NANOTUBE MANUFACTURING AND APPLICATIONS" FINAL REPORT (PDF) (Report). World Technology Evaluation Center(WTEC).
- ^ M.Endo, "Grow carbonfibers in the vapor phase", ChemTech, 18, no.9, pp.568-576(1988)
- ^ Koyama, T. and Endo, M.T. (1983) "Method for Manufacturing Carbon Fibers by a Vapor Phase Process," Japanese Patent, 1982-58, 966.
- ^ Abrahamson, John; Wiles, Peter G.; Rhoades, Brian L. (1999). "Structure of Carbon Fibers Found on Carbon Arc Anodes". Carbon. 37 (11): 1873–1874. doi:10.1016/S0008-6223(99)00199-2.
- ^ Izvestiya Akademii Nauk SSSR, Metals. 1982, #3, pp.12–17 (in Russian)
- ^ US 4663230, Tennent, Howard G., "Carbon fibrils, method for producing same and compositions containing same", issued 1987-05-05
- ^ Iijima, Sumio (7 November 1991). "Helical microtubules of graphitic carbon". Nature. 354 (6348): 56–58. Bibcode:1991Natur.354...56I. doi:10.1038/354056a0.
- ^ Mintmire, J.W.; Dunlap, BI; White, CT (1992). "Are Fullerene Tubules Metallic?". Phys. Rev. Lett. 68 (5): 631–634. Bibcode:1992PhRvL..68..631M. doi:10.1103/PhysRevLett.68.631. PMID 10045950.
- ^ Bethune, D. S.; Kiang, C. H.; De Vries, M. S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. (1993). "Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls". Nature. 363 (6430): 605–607. Bibcode:1993Natur.363..605B. doi:10.1038/363605a0.
- ^ Iijima, Sumio; Ichihashi, Toshinari (1993). "Single-shell carbon nanotubes of 1-nm diameter". Nature. 363 (6430): 603–605. Bibcode:1993Natur.363..603I. doi:10.1038/363603a0.
- ^ Krätschmer, W.; Lamb, Lowell D.; Fostiropoulos, K.; Huffman, Donald R. (1990). "Solid C60: a new form of carbon". Nature. 347 (6291): 354–358. Bibcode:1990Natur.347..354K. doi:10.1038/347354a0.
External links
This article's use of external links may not follow Wikipedia's policies or guidelines. (September 2015) |
- Nanohedron.com image gallery with carbon nanotubes
- The Nanotube site. Last updated 2009.05.03
- EU Marie Curie Network CARBIO: Multifunctional carbon nanotubes for biomedical applications
- Carbon nanotube on arxiv.org
- C60 and Carbon Nanotubes a short video explaining how nanotubes can be made from modified graphite sheets and the three different types of nanotubes that are formed
- Carbon Nanotubes & Buckyballs.
- The Wondrous World of Carbon Nanotubes
- Learning module for Bandstructure of Carbon Nanotubes and Nanoribbons
- Durability of carbon nanotubes and their potential to cause inflammation by Dr Megan Osmond and others. (SafeWork Australia, May 2011). This was a collaboration between the Institute of Occupational Medicine, Edinburgh University and CSIRO in Australia.
- NT06 Seventh International Conference on the Science and Application of Nanotubes
- NT05 Sixth International Conference on the Science and Application of Nanotubes
- Selection of free-download articles on carbon nanotubes
- First computer made of carbon nanotubes is unveiled (BBC News) 2013-09-25
- Research using carbon nanotubes for microfabrication and in other applications