Fermentation


Fermentation is a metabolic process that converts sugar to acids, gases and/or alcohol. It occurs in yeast and bacteria, but also in oxygen-starved muscle cells, as in the case of lactic acid fermentation. Fermentation is also used more broadly to refer to the bulk growth of microorganisms on a growth medium. French microbiologist Louis Pasteur is often remembered for his insights into fermentation and its microbial causes. The science of fermentation is known as zymology.
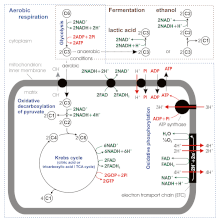
Fermentation takes place in the absence of oxygen (when the electron transport chain is unusable) and becomes the cell’s primary means of ATP (energy) production.[1] It turns NADH and pyruvate produced in the glycolysis step into NAD+ and various small molecules (see examples below). In the presence of O2, NADH and pyruvate are used in respiration; this is oxidative phosphorylation, it generates a lot more ATP in addition to that created by glycolysis, and for that reason cells generally benefit from avoiding fermentation when oxygen is available. Exceptions include obligate anaerobes, which cannot tolerate oxygen.
The first step, glycolysis, is common to all fermentation pathways:
- C6H12O6 + 2 NAD+ + 2 ADP + 2 Pi → 2 CH3COCOO− + 2 NADH + 2 ATP + 2 H2O + 2H+
Pyruvate is CH3COCOO−. Pi is phosphate. Two ADP molecules and two Pi are converted to two ATP and two water molecules via substrate-level phosphorylation. Two molecules of NAD+ are also reduced to NADH.[2]
In oxidative phosphorylation the energy for ATP formation is derived from an electrochemical proton gradient generated across the inner mitochondrial membrane (or, in the case of bacteria, the plasma membrane) via the electron transport chain. Glycolysis has substrate-level phosphorylation (ATP generated directly at the point of reaction).
Fermentation has been used by humans for the production of food and beverages since the Neolithic age. For example, fermentation is employed for preservation in a process that produces lactic acid as found in such sour foods as pickled cucumbers, kimchi and yogurt (see fermentation in food processing), as well as for producing alcoholic beverages such as wine (see fermentation in winemaking) and beer. Fermentation can even occur within the stomachs of animals, such as humans. Auto-brewery syndrome is a rare medical condition where the stomach contains brewers yeast that break down starches into ethanol; which enters the blood stream.[3]
Examples
Fermentation does not necessarily have to be carried out in an anaerobic environment. For example, even in the presence of abundant oxygen, yeast cells greatly prefer fermentation to aerobic respiration, as long as sugars are readily available for consumption (a phenomenon known as the Crabtree effect).[4] The antibiotic activity of hops also inhibits aerobic metabolism in yeast.
Fermentation reacts NADH with an endogenous, organic electron acceptor.[1] Usually this is pyruvate formed from the sugar during the glycolysis step. During fermentation, pyruvate is metabolized to various compounds through several processes:
- ethanol fermentation, aka alcoholic fermentation, is the production of ethanol and carbon dioxide
- lactic acid fermentation refers to two means of producing lactic acid:
- homolactic fermentation is the production of lactic acid exclusively
- heterolactic fermentation is the production of lactic acid as well as other acids and alcohols.
Sugars are the most common substrate of fermentation, and typical examples of fermentation products are ethanol, lactic acid, carbon dioxide, and hydrogen gas (H2). However, more exotic compounds can be produced by fermentation, such as butyric acid and acetone. Yeast carries out fermentation in the production of ethanol in beers, wines, and other alcoholic drinks, along with the production of large quantities of carbon dioxide. Fermentation occurs in mammalian muscle during periods of intense exercise where oxygen supply becomes limited, resulting in the creation of lactic acid.[5]
Chemistry
Fermentation products contain chemical energy (they are not fully oxidized), but are considered waste products, since they cannot be metabolized further without the use of oxygen.
Ethanol fermentation
The chemical equation below shows the alcoholic fermentation of glucose, whose chemical formula is C6H12O6.[7] One glucose molecule is converted into two ethanol molecules and two carbon dioxide molecules:
- C6H12O6 → 2 C2H5OH + 2 CO2
C2H5OH is the chemical formula for ethanol.
Before fermentation takes place, one glucose molecule is broken down into two pyruvate molecules. This is known as glycolysis.[7][8]
Lactic acid fermentation
Homolactic fermentation (producing only lactic acid) is the simplest type of fermentation. The pyruvate from glycolysis[9] undergoes a simple redox reaction, forming lactic acid.[2][10] It is unique because it is one of the only respiration processes to not produce a gas as a byproduct.
Overall, one molecule of glucose (or any six-carbon sugar) is converted to two molecules of lactic acid: C6H12O6 → 2 CH3CHOHCOOH
It occurs in the muscles of animals when they need energy faster than the blood can supply oxygen. It also occurs in some kinds of bacteria (such as lactobacilli) and some fungi. It is this type of bacteria that converts lactose into lactic acid in yogurt, giving it its sour taste. These lactic acid bacteria can carry out either homolactic fermentation, where the end-product is mostly lactic acid, or
Heterolactic fermentation, where some lactate is further metabolized and results in ethanol and carbon dioxide[2] (via the phosphoketolase pathway[11]), acetate, or other metabolic products, e.g: C6H12O6 → CH3CHOHCOOH + C2H5OH + CO2
If lactose is fermented (as in yogurts and cheeses), it is first converted into glucose and galactose (both six-carbon sugars with the same atomic formula): C12H22O11 + H2O → 2 C6H12O6
Heterolactic fermentation is in a sense intermediate between lactic acid fermentation, and other types, e.g alcoholic fermentation (see below). The reasons to go further and convert lactic acid into anything else are:
- The acidity of lactic acid impedes biological processes; this can be beneficial to the fermenting organism as it drives out competitors who are unadapted to the acidity; as a result the food will have a longer shelf-life (part of the reason foods are purposely fermented in the first place); however, beyond a certain point, the acidity starts affecting the organism that produces it.
- The high concentration of lactic acid (the final product of fermentation) drives the equilibrium backwards (Le Chatelier's principle), decreasing the rate at which fermentation can occur, and slowing down growth
- Ethanol, that lactic acid can be easily converted to, is volatile and will readily escape, allowing the reaction to procede easily. CO2 is also produced, however it's only weakly acidic, and even more volatile than ethanol.
- Acetic acid (another conversion product) is acidic, and not as volatile as ethanol; however, in the presence of limited oxygen, its creation from lactic acid releases a lot of additional energy. It is a lighter molecule than lactic acid, that forms fewer hydrogen bonds with its surroundings (due to having fewer groups that can form such bonds), and thus more volatile and will also allow the reaction to move forward more quickly.
- If propionic acid, butyric acid and longer monocarboxylic acids are produced (see mixed acid fermentation), the amount of acidity produced per glucose consumed will decrease, as with ethanol, allowing faster growth.
Aerobic respiration
In aerobic respiration, the pyruvate produced by glycolysis is oxidized completely, generating additional ATP and NADH in the citric acid cycle and by oxidative phosphorylation. However, this can occur only in the presence of oxygen. Oxygen is toxic to organisms that are obligate anaerobes, and is not required by facultative anaerobic organisms. In the absence of oxygen, one of the fermentation pathways occurs in order to regenerate NAD+; lactic acid fermentation is one of these pathways.[2]
Hydrogen gas production in fermentation
Hydrogen gas is produced in many types of fermentation (mixed acid fermentation, butyric acid fermentation, caproate fermentation, butanol fermentation, glyoxylate fermentation), as a way to regenerate NAD+ from NADH. Electrons are transferred to ferredoxin, which in turn is oxidized by hydrogenase, producing H2.[7] Hydrogen gas is a substrate for methanogens and sulfate reducers, which keep the concentration of hydrogen low and favor the production of such an energy-rich compound,[12] but hydrogen gas at a fairly high concentration can nevertheless be formed, as in flatus.
As an example of mixed acid fermentation, bacteria such as Clostridium pasteurianum ferment glucose producing butyrate, acetate, carbon dioxide and hydrogen gas:[13] The reaction leading to acetate is:
- C6H12O6 + 4 H2O → 2 CH3COO- + 2 HCO3- + 4 H+ + 4 H2
Glucose could theoretically be converted into just CO2 and H2, but the global reaction releases little energy.
Methane gas production in fermentation
Acetic acid can also undergo a dismutation reaction to produce methane and carbon dioxide:[14][15]
- CH3COO– + H+ → CH4 + CO2 ΔG° = -36 kJ/reaction
This disproportionation reaction is catalysed by methanogen archaea in their fermentative metabolism. One electron is transferred from the carbonyl function (e– donor) of the carboxylic group to the methyl group (e– acceptor) of acetic acid to respectively produce CO2 and methane gas.
History
The use of fermentation, particularly for beverages, has existed since the Neolithic and has been documented dating from 7000–6600 BCE in Jiahu, China,[16] 6000 BCE in Georgia,[17] 3150 BCE in ancient Egypt,[18] 3000 BCE in Babylon,[19] 2000 BCE in pre-Hispanic Mexico,[19] and 1500 BC in Sudan.[20] Fermented foods have a religious significance in Judaism and Christianity. The Baltic god Rugutis was worshiped as the agent of fermentation.[21][22]
The first solid evidence of the living nature of yeast appeared between 1837 and 1838 when three publications appeared by C. Cagniard de la Tour, T. Swann, and F. Kuetzing, each of whom independently concluded as a result of microscopic investigations that yeast is a living organism that reproduces by budding. It is perhaps because wine, beer, and bread were each basic foods in Europe that most of the early studies on fermentation were done on yeasts, with which they were made. Soon, bacteria were also discovered; the term was first used in English in the late 1840s, but it did not come into general use until the 1870s, and then largely in connection with the new germ theory of disease.[23]
Louis Pasteur (1822–1895), during the 1850s and 1860s, showed that fermentation is initiated by living organisms in a series of investigations.[10] In 1857, Pasteur showed that lactic acid fermentation is caused by living organisms.[24] In 1860, he demonstrated that bacteria cause souring in milk, a process formerly thought to be merely a chemical change, and his work in identifying the role of microorganisms in food spoilage led to the process of pasteurization.[25] In 1877, working to improve the French brewing industry, Pasteur published his famous paper on fermentation, "Etudes sur la Bière", which was translated into English in 1879 as "Studies on fermentation".[26] He defined fermentation (incorrectly) as "Life without air",[27] but correctly showed that specific types of microorganisms cause specific types of fermentations and specific end-products.
Although showing fermentation to be the result of the action of living microorganisms was a breakthrough, it did not explain the basic nature of the fermentation process, or prove that it is caused by the microorganisms that appear to be always present. Many scientists, including Pasteur, had unsuccessfully attempted to extract the fermentation enzyme from yeast.[27] Success came in 1897 when the German chemist Eduard Buechner ground up yeast, extracted a juice from them, then found to his amazement that this "dead" liquid would ferment a sugar solution, forming carbon dioxide and alcohol much like living yeasts.[28] The "unorganized ferments" behaved just like the organized ones. From that time on, the term enzyme came to be applied to all ferments. It was then understood that fermentation is caused by enzymes that are produced by microorganisms.[29] In 1907, Buechner won the Nobel Prize in chemistry for his work.[30]
Advances in microbiology and fermentation technology have continued steadily up until the present. For example, in the late 1970s, it was discovered that microorganisms could be mutated with physical and chemical treatments to be higher-yielding, faster-growing, tolerant of less oxygen, and able to use a more concentrated medium.[31] Strain selection and hybridization developed as well, affecting most modern food fermentations.
Etymology
The word ferment is derived from the Latin verb fervere, which means to boil (same root as effervescence). It is thought to have been first used in the late fourteenth century in alchemy, but only in a broad sense. It was not used in the modern scientific sense until around 1600.[32] The word yeast is derived from *jes- the PIE word meaning boil (cf. Greek zein, Welsh ias, and Sanskrit yasyati).[33]
See also
References
- ^ a b Klein, Donald W.; Lansing M.; Harley, John (2006). Microbiology (6th ed.). New York: McGraw-Hill. ISBN 978-0-07-255678-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d AP Biology. Anestis, Mark. 2nd Edition. McGraw-Hill Professional. 2006. ISBN 978-0-07-147630-0. p. 61
- ^ Barbara Cordell; Justin McCarthy (July 2013). "A Case Study of Gut Fermentation Syndrome (Auto-Brewery) with Saccharomyces cerevisiae as the Causative Organism". International Journal of Clinical Medicine. 4: 309–312.
- ^ Dickinson, J. R. (1999). "Carbon metabolism". In J. R. Dickinson and M. Schweizer (ed.). The metabolism and molecular physiology of Saccharomyces cerevisiae. Philadelphia, PA: Taylor & Francis. ISBN 978-0-7484-0731-6.
- ^
Voet, Donald & Voet, Judith G. (1995). Biochemistry (2nd ed.). New York, NY: John Wiley & Sons. ISBN 978-0-471-58651-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Stryer, Lubert (1995). Biochemistry (fourth ed.). New York - Basingstoke: W. H. Freeman and Company. ISBN 978-0716720096.
- ^ a b c Life, the science of biology. Purves, William Kirkwood. Sadava, David. Orians, Gordon H. 7th Edition. Macmillan Publishers. 2004. ISBN 978-0-7167-9856-9. pp. 139–140
- ^ Stryer, Lubert (1975). Biochemistry. W. H. Freeman and Company. ISBN 0-7167-0174-X.
- ^ Introductory Botany: plants, people, and the Environment. Berg, Linda R. Cengage Learning, 2007. ISBN 978-0-534-46669-5. p. 86
- ^ a b A dictionary of applied chemistry, Volume 3. Thorpe, Sir Thomas Edward. Longmans, Green and Co., 1922. p.159
- ^ Cite error: The named reference
fao1was invoked but never defined (see the help page). - ^ Madigan, Michael T.; Martinko, John M.; Parker, Jack (1996). Brock biology of microorganisms (8th ed.). Prentice Hall. ISBN 978-0-13-520875-5.
- ^ Thauer, R.K.; Jungermann, K.; Decker, K. (1977). "Energy conservation in chemotrophic anaerobic bacteria". Bacteriological Reviews. 41 (1): 100–80. ISSN 0005-3678. PMC 413997. PMID 860983.
- ^ Ferry, J.G. (1992). "Methane from acetate". Journal of Bacteriology. 174 (17): 5489–5495. PMC 206491. PMID 1512186. Retrieved 2011-11-05.
- ^ Vogels, G.D. (1988). "Biochemistry of methane production". In Zehnder A.J.B. (ed.). Biology of anaerobic microorganisms. New York: Wiley. pp. 707–770.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1073/pnas.0407921102, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1073/pnas.0407921102instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1079/PGR2006114, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1079/PGR2006114instead. - ^ Cavalieri, D (2003). "Evidence for S. cerevisiae fermentation in ancient wine" (PDF). Journal of Molecular Evolution. 57 Suppl 1: S226–32. doi:10.1007/s00239-003-0031-2. PMID 15008419. 15008419. Archived from the original (PDF) on April 17, 2007. Retrieved 2007-01-28.
{{cite journal}}: Cite has empty unknown parameter:|quotes=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b "Fermented fruits and vegetables. A global perspective". FAO Agricultural Services Bulletins - 134. Archived from the original on January 19, 2007. Retrieved 2007-01-28.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Dirar, H., (1993), The Indigenous Fermented Foods of the Sudan: A Study in African Food and Nutrition, CAB International, UK
- ^ http://www.spauda.lt/mitai/lietuva/lietdiev.htm
- ^ Rūgutis. Mitologijos enciklopedija, 2 tomas. Vilnius. Vaga. 1999. 293 p.
- ^ A brief history of fermentation, East and West. Soyinfocenter.com. Retrieved on 2011-01-04.
- ^ Accomplishments of Louis Pasteur. Fjcollazo.com (2005-12-30). Retrieved on 2011-01-04.
- ^ HowStuffWorks "Louis Pasteur". Science.howstuffworks.com (2009-07-01). Retrieved on 2011-01-04.
- ^ Louis Pasteur (1879) Studies on fermentation: The diseases of beer, their causes, and the means of preventing them. Macmillan Publishers.
- ^ a b Modern History Sourcebook: Louis Pasteur (1822–1895): Physiological theory of fermentation, 1879. Translated by F. Faulkner, D.C. Robb.
- ^ New beer in an old bottle: Eduard Buchner and the Growth of Biochemical Knowledge. Cornish-Bowden, Athel. Universitat de Valencia. 1997. ISBN 978-84-370-3328-0. p. 25.
- ^ The enigma of ferment: from the philosopher’s stone to the first biochemical Nobel prize. Lagerkvist, Ulf. World Scientific Publishers. 2005. ISBN 978-981-256-421-4. p. 7.
- ^ A treasury of world science, Volume 1962, Part 1. Runes, Dagobert David. Philosophical Library Publishers. 1962. p. 109.
- ^ Wang, H. L.; Swain, E. W.; Hesseltine, C. W. (1980). "Phytase of molds used in oriental food fermentation". Journal of Food Science. 45 (5): 1262. doi:10.1111/j.1365-2621.1980.tb06534.x.
- ^ Fermentation | Define fermentation at Dictionary.com. Dictionary.reference.com. Retrieved on 2011-01-04.
- ^ "Yeast". Etymonline.