Penem
A penem is a type of unsaturated β-lactam.
Penems are similar in structure to carbapenems. However, where penems have a sulfur, carbapenems have another carbon.[2]
There are no known naturally occurring penems; all of them are synthetically made.
Structure

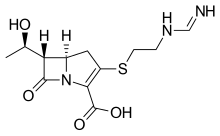

Penem molecules do not occur naturally, and production of penems is an entirely synthetic process.
Although structurally distinct, the penems are often confused with the carbapenem class of drugs.
Five main penem subgroups — thiopenems, oxypenems, aminopenems, alkylpenems, and arylpenems — have been produced and are distinguished by the side chain (at position 2) of the unsaturated five-membered ring. One structurally distinct penem is BRL 42715. This molecule has no substitution at the above position, but has a bulky group attached to the β-lactam ring, and it displays effective inhibition of class C β-lactamases, but no antimicrobial activity.
One possible consequence of these structural differences of penems from other β-lactams may be reduced immunogenicity and immunogenic cross-reactivity
References
- ^ Milazzo I, Blandino G, Caccamo F, Musumeci R, Nicoletti G, Speciale A (March 2003). "Faropenem, a new oral penem: antibacterial activity against selected anaerobic and fastidious periodontal isolates". Journal of antimicrobial chemotherapy. 51 (3): 721–5. doi:10.1093/jac/dkg120. PMID 12615878.
- ^ "Medscape.com".
Further reading
- Sasaki A, Goda K, Enomoto M, Sunagawa M (May 1992). "Synthetic studies of carbapenem and penem antibiotics. II. Synthesis of 3-acetyl-2-azetidinones by (2 + 2) cycloaddition of diketene and Schiff bases". Chemical & pharmaceutical bulletin. 40 (5): 1094–7. doi:10.1248/cpb.40.1094. PMID 1394625.
