Population genetics
| Part of a series on |
| Evolutionary biology |
|---|
 |
Population genetics is the study of the distribution and change in frequency of alleles within populations, and as such it sits firmly within the field of evolutionary biology. The main processes of evolution are natural selection, genetic drift, gene flow, mutation, and genetic recombination and they form an integral part of the theory that underpins population genetics. Studies in this branch of biology examine such phenomena as adaptation, speciation, population subdivision, and population structure.
Population genetics was a vital ingredient in the emergence of the modern evolutionary synthesis. Its primary founders were Sewall Wright, J. B. S. Haldane and Ronald Fisher, who also laid the foundations for the related discipline of quantitative genetics.
Traditionally a highly mathematical discipline, modern population genetics encompasses theoretical, lab and field work. Computational approaches, often utilising coalescent theory, have played a central role since the 1980s.
History

Population genetics began as a reconciliation of Mendelian inheritance and biostatistics models. A key step was the work of the British biologist and statistician Ronald Fisher. In a series of papers starting in 1918 and culminating in his 1930 book The Genetical Theory of Natural Selection, Fisher showed that the continuous variation measured by the biometricians could be produced by the combined action of many discrete genes, and that natural selection could change allele frequencies in a population, resulting in evolution. In a series of papers beginning in 1924, another British geneticist, J.B.S. Haldane worked out the mathematics of allele frequency change at a single gene locus under a broad range of conditions. Haldane also applied statistical analysis to real-world examples of natural selection, such as the Peppered moth evolution and industrial melanism, and showed that selection coefficients could be larger than Fisher assumed, leading to more rapid adaptive evolution.[1][2]
The American biologist Sewall Wright, who had a background in animal breeding experiments, focused on combinations of interacting genes, and the effects of inbreeding on small, relatively isolated populations that exhibited genetic drift. In 1932, Wright introduced the concept of an adaptive landscape and argued that genetic drift and inbreeding could drive a small, isolated sub-population away from an adaptive peak, allowing natural selection to drive it towards different adaptive peaks.
The work of Fisher, Haldane and Wright founded the discipline of population genetics. This integrated natural selection with Mendelian genetics, which was the critical first step in developing a unified theory of how evolution worked.[1][2] John Maynard Smith was Haldane's pupil, whilst W.D. Hamilton was heavily influenced by the writings of Fisher. The American George R. Price worked with both Hamilton and Maynard Smith. American Richard Lewontin and Japanese Motoo Kimura were heavily influenced by Wright.
Modern evolutionary synthesis
The mathematics of population genetics were originally developed as the beginning of the modern evolutionary synthesis. According to Beatty (1986), population genetics defines the core of the modern synthesis. In the first few decades of the 20th century, most field naturalists continued to believe that Lamarckian and orthogenic mechanisms of evolution provided the best explanation for the complexity they observed in the living world. However, as the field of genetics continued to develop, those views became less tenable.[3] During the modern evolutionary synthesis, these ideas were purged, and only evolutionary causes that could be expressed in the mathematical framework of population genetics were retained.[4] Consensus was reached as to which evolutionary factors might influence evolution, but not as to the relative importance of the various factors.[4]
Theodosius Dobzhansky, a postdoctoral worker in T. H. Morgan's lab, had been influenced by the work on genetic diversity by Russian geneticists such as Sergei Chetverikov. He helped to bridge the divide between the foundations of microevolution developed by the population geneticists and the patterns of macroevolution observed by field biologists, with his 1937 book Genetics and the Origin of Species. Dobzhansky examined the genetic diversity of wild populations and showed that, contrary to the assumptions of the population geneticists, these populations had large amounts of genetic diversity, with marked differences between sub-populations. The book also took the highly mathematical work of the population geneticists and put it into a more accessible form. Many more biologists were influenced by population genetics via Dobzhansky than were able to read the highly mathematical works in the original.[5]
Selection vs. genetic drift
Fisher and Wright had some fundamental disagreements about the relative roles of selection and drift.[6]
In Great Britain E.B. Ford, the pioneer of ecological genetics, continued throughout the 1930s and 1940s to demonstrate the power of selection due to ecological factors including the ability to maintain genetic diversity through genetic polymorphisms such as human blood types. Ford's work, in collaboration with Fisher, contributed to a shift in emphasis during the course of the modern synthesis towards natural selection over genetic drift.[1][2][7][8]
Recent studies of eukaryotic transposable elements, and of their impact on speciation, point again to a major role of nonadaptive processes such as mutation and genetic drift.[9] Mutation and genetic drift are also viewed as major factors in the evolution of genome complexity.[10]
Fundamentals
Population genetics is the study of the frequency and interaction of alleles and genes in populations.[11] A sexual population is a set of organisms in which any pair of members can breed freely together. This implies that all members belong to the same species and are located near each other.[12]
For example, all of the moths of the same species living in an isolated forest are a population. A gene in this population may have several alternate forms, which account for variations between the phenotypes of the organisms. An example might be a gene for coloration in moths that has two alleles: black and white. A gene pool is the complete set of alleles for a gene in a single population; the allele frequency for an allele is the fraction of the genes in the pool that is composed of that allele (for example, what fraction of moth coloration genes are the black allele). Evolution occurs when there are changes in the frequencies of alleles within a population; for example, the allele for black color in a population of moths becoming more common.
| Part of a series on |
| Genetic genealogy |
|---|
| Concepts |
| Related topics |
Four processes
Selection
Natural selection, which includes sexual selection, is the fact that some traits make it more likely for an organism to survive and reproduce. Population genetics describes natural selection by defining fitness as a propensity or probability of survival and reproduction in a particular environment. The fitness is normally given by the symbol w=1-s where s is the selection coefficient. Natural selection acts on phenotypes, or the observable characteristics of organisms, but the genetically heritable basis of any phenotype which gives a reproductive advantage will become more common in a population (see allele frequency). In this way, natural selection converts differences in fitness into changes in allele frequency in a population over successive generations.
Before the advent of population genetics, many biologists doubted that small differences in fitness were sufficient to make a large difference to evolution.[5] Population geneticists addressed this concern in part by comparing selection to genetic drift. Selection can overcome genetic drift when s is greater than 1 divided by the effective population size. When this criterion is met, the probability that a new advantageous mutant becomes fixed is approximately equal to 2s.[13][14] The time until fixation of such an allele depends little on genetic drift, and is approximately proportional to log(sN)/s.[15]
Hardy–Weinberg principle
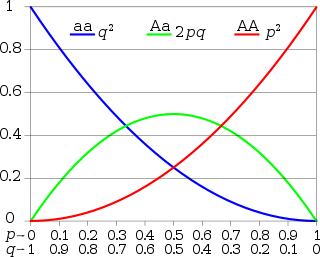
Natural selection will only cause evolution if there is enough genetic variation in a population. Before the discovery of Mendelian genetics, one common hypothesis was blending inheritance. But with blending inheritance, genetic variance would be rapidly lost, making evolution by natural or sexual selection implausible. The Hardy–Weinberg principle provides the solution to how variation is maintained in a population with Mendelian inheritance. According to this principle, the frequencies of alleles (variations in a gene) will remain constant in the absence of selection, mutation, migration and genetic drift.[16] The Hardy–Weinberg "equilibrium" refers to this stability of allele frequencies over time.
A second component of the Hardy–Weinberg principle concerns the effects of a single generation of random mating. In this case, the genotype frequencies can be predicted from the allele frequencies. For example, in the simplest case of a single locus with two alleles: the dominant allele is denoted A and the recessive a and their frequencies are denoted by p and q; freq(A) = p; freq(a) = q; p + q = 1. If the genotype frequencies are in Hardy–Weinberg proportions resulting from random mating, then we will have freq(AA) = p2 for the AA homozygotes in the population, freq(aa) = q2 for the aa homozygotes, and freq(Aa) = 2pq for the heterozygotes.
Genetic drift
Genetic drift is a change in allele frequencies caused by random sampling.[17] That is, the alleles in the offspring are a random sample of those in the parents.[18] Genetic drift may cause gene variants to disappear completely, and thereby reduce genetic variability. In contrast to natural selection, which makes gene variants more common or less common depending on their reproductive success,[19] the changes due to genetic drift are not driven by environmental or adaptive pressures, and may be beneficial, neutral, or detrimental to reproductive success.
The effect of genetic drift is larger for alleles present in few copies than when an allele is present in many copies. Scientists wage vigorous debates over the relative importance of genetic drift compared with natural selection. Ronald Fisher held the view that genetic drift plays at the most a minor role in evolution, and this remained the dominant view for several decades. In 1968 Motoo Kimura rekindled the debate with his neutral theory of molecular evolution which claims that most of the changes in the genetic material are caused by neutral mutations and genetic drift.[20] The role of genetic drift by means of sampling error in evolution has been criticized by John H Gillespie[21] and Will Provine,[22] who argue that selection on linked sites is a more important stochastic force.
The population genetics of genetic drift are described using either branching processes or a diffusion equation describing changes in allele frequency.[23] These approaches are usually applied to the Wright-Fisher and John Moran models of population genetics. Assuming genetic drift is the only evolutionary force acting on an allele, after t generations in many replicated populations, starting with allele frequencies of p and q, the variance in allele frequency across those populations is
Mutation

Mutation is the ultimate source of genetic variation in the form of new alleles. Mutation can result in several different types of change in DNA sequences; these can either have no effect, alter the product of a gene, or prevent the gene from functioning. Studies in the fly Drosophila melanogaster suggest that if a mutation changes a protein produced by a gene, this will probably be harmful, with about 70 percent of these mutations having damaging effects, and the remainder being either neutral or weakly beneficial.[25]
Mutations can involve large sections of DNA becoming duplicated, usually through genetic recombination.[26] These duplications are a major source of raw material for evolving new genes, with tens to hundreds of genes duplicated in animal genomes every million years.[27] Most genes belong to larger families of homologous shared ancestry.[28] Novel genes are produced by several methods, commonly through the duplication and mutation of an ancestral gene, or by recombining parts of different genes to form new combinations with new functions.[29][30] Here, protein domains act as modules, each with a particular and independent function, that can be mixed together to produce genes encoding new proteins with novel properties.[31] For example, the human eye uses four genes to make structures that sense light: three for the cone cell which produce color vision and one for the rod cell which produces night vision; all four arose from a single ancestral gene.[32] Another advantage of duplicating a gene (or even an entire genome) is that this increases redundancy; this allows one gene in the pair to acquire a new function while the other copy performs the original function.[33][34] Other types of mutation occasionally create new genes from previously noncoding DNA.[35][36]
In addition to being a major source of variation, mutation may also function as a mechanism of evolution when there are different probabilities at the molecular level for different mutations to occur, a process known as mutation bias.[37] If two genotypes, for example one with the nucleotide G and another with the nucleotide A in the same position, have the same fitness, but mutation from G to A happens more often than mutation from A to G, then genotypes with A will tend to evolve.[38] Different insertion vs. deletion mutation biases in different taxa can lead to the evolution of different genome sizes.[39][40] Developmental or mutational biases have also been observed in morphological evolution.[41][42] For example, according to the phenotype-first theory of evolution, mutations can eventually cause the genetic assimilation of traits that were previously induced by the environment.[43][44]
Mutation bias effects are superimposed on other processes. If selection would favor either one out of two mutations, but there is no extra advantage to having both, then the mutation that occurs the most frequently is the one that is most likely to become fixed in a population.[45][46] Mutations leading to the loss of function of a gene are much more common than mutations that produce a new, fully functional gene. Most loss of function mutations are selected against. But when selection is weak, mutation bias towards loss of function can affect evolution.[47] For example, pigments are no longer useful when animals live in the darkness of caves, and tend to be lost.[48] This kind of loss of function can occur because of mutation bias, and/or because the function had a cost, and once the benefit of the function disappeared, natural selection leads to the loss. Loss of sporulation ability in a bacterium during laboratory evolution appears to have been caused by mutation bias, rather than natural selection against the cost of maintaining sporulation ability.[49] When there is no selection for loss of function, the speed at which loss evolves depends more on the mutation rate than it does on the effective population size,[50] indicating that it is driven more by mutation bias than by genetic drift.
Evolution of mutation rate
Due to the damaging effects that mutations can have on cells, organisms have evolved mechanisms such as DNA repair to remove mutations.[51] Therefore, the optimal mutation rate for a species may be trade-off between costs of a high mutation rate, such as deleterious mutations, and the metabolic costs of maintaining systems to reduce the mutation rate, such as DNA repair enzymes.[52] Viruses that use RNA as their genetic material have rapid mutation rates,[53] which can be an advantage since these viruses will evolve constantly and rapidly, and thus evade the defensive responses of e.g. the human immune system.[54]
Gene flow and transfer


Gene flow is the exchange of genes between populations, which are usually of the same species.[55] Examples of gene flow within a species include the migration and then breeding of organisms, or the exchange of pollen. Gene transfer between species includes the formation of hybrid organisms and horizontal gene transfer.
Migration into or out of a population can change allele frequencies, as well as introducing genetic variation into a population. Immigration may add new genetic material to the established gene pool of a population. Conversely, emigration may remove genetic material. Population genetic models can be used to reconstruct the history of gene flow between populations.[56]
Reproductive isolation

As barriers to reproduction between two diverging populations are required for the populations to become new species, gene flow may slow this process by spreading genetic differences between the populations. Gene flow is hindered by mountain ranges, oceans and deserts or even man-made structures such as the Great Wall of China, which has hindered the flow of plant genes.[57]
Depending on how far two species have diverged since their most recent common ancestor, it may still be possible for them to produce offspring, as with horses and donkeys mating to produce mules.[58] Such hybrids are generally infertile, due to the two different sets of chromosomes being unable to pair up during meiosis. In this case, closely related species may regularly interbreed, but hybrids will be selected against and the species will remain distinct. However, viable hybrids are occasionally formed and these new species can either have properties intermediate between their parent species, or possess a totally new phenotype.[59] The importance of hybridization in creating new species of animals is unclear, although cases have been seen in many types of animals,[60] with the gray tree frog being a particularly well-studied example.[61]
Hybridization is, however, an important means of speciation in plants, since polyploidy (having more than two copies of each chromosome) is tolerated in plants more readily than in animals.[62][63] Polyploidy is important in hybrids as it allows reproduction, with the two different sets of chromosomes each being able to pair with an identical partner during meiosis.[64] Polyploids also have more genetic diversity, which allows them to avoid inbreeding depression in small populations.[65]
Genetic structure
Because of physical barriers to migration, along with limited tendency for individuals to move or spread (vagility), and tendency to remain or come back to natal place (philopatry), natural populations rarely all interbreed as convenient in theoretical random models (panmixy) (Buston et al., 2007). There is usually a geographic range within which individuals are more closely related to one another than those randomly selected from the general population. This is described as the extent to which a population is genetically structured (Repaci et al., 2007). Genetic structuring can be caused by migration due to historical climate change, species range expansion or current availability of habitat.
Horizontal Gene Transfer

Horizontal gene transfer is the transfer of genetic material from one organism to another organism that is not its offspring; this is most common among bacteria.[66] In medicine, this contributes to the spread of antibiotic resistance, as when one bacteria acquires resistance genes it can rapidly transfer them to other species.[67] Horizontal transfer of genes from bacteria to eukaryotes such as the yeast Saccharomyces cerevisiae and the adzuki bean beetle Callosobruchus chinensis may also have occurred.[68][69] An example of larger-scale transfers are the eukaryotic bdelloid rotifers, which appear to have received a range of genes from bacteria, fungi, and plants.[70] Viruses can also carry DNA between organisms, allowing transfer of genes even across biological domains.[71] Large-scale gene transfer has also occurred between the ancestors of eukaryotic cells and prokaryotes, during the acquisition of chloroplasts and mitochondria.[72]
Complications
Basic models of population genetics consider only one gene locus at a time. In practice, epistatic and linkage relationships between loci may also be important.
Epistasis
Because of epistasis, the phenotypic effect of an allele at one locus may depend on which alleles are present at many other loci. Selection does not act on a single locus, but on a phenotype that arises through development from a complete genotype.
According to Lewontin (1974), the theoretical task for population genetics is a process in two spaces: a "genotypic space" and a "phenotypic space". The challenge of a complete theory of population genetics is to provide a set of laws that predictably map a population of genotypes (G1) to a phenotype space (P1), where selection takes place, and another set of laws that map the resulting population (P2) back to genotype space (G2) where Mendelian genetics can predict the next generation of genotypes, thus completing the cycle. Even leaving aside for the moment the non-Mendelian aspects of molecular genetics, this is clearly a gargantuan task. Visualizing this transformation schematically:
(adapted from Lewontin 1974, p. 12). XD
T1 represents the genetic and epigenetic laws, the aspects of functional biology, or development, that transform a genotype into phenotype. We will refer to this as the "genotype-phenotype map". T2 is the transformation due to natural selection, T3 are epigenetic relations that predict genotypes based on the selected phenotypes and finally T4 the rules of Mendelian genetics.
In practice, there are two bodies of evolutionary theory that exist in parallel, traditional population genetics operating in the genotype space and the biometric theory used in plant and animal breeding, operating in phenotype space. The missing part is the mapping between the genotype and phenotype space. This leads to a "sleight of hand" (as Lewontin terms it) whereby variables in the equations of one domain, are considered parameters or constants, where, in a full-treatment they would be transformed themselves by the evolutionary process and are in reality functions of the state variables in the other domain. The "sleight of hand" is assuming that we know this mapping. Proceeding as if we do understand it is enough to analyze many cases of interest. For example, if the phenotype is almost one-to-one with genotype (sickle-cell disease) or the time-scale is sufficiently short, the "constants" can be treated as such; however, there are many situations where it is inaccurate.
Linkage
If all genes are in linkage equilibrium, the effect of an allele at one locus can be averaged across the gene pool at other loci. In reality, one allele is frequently found in linkage disequilibrium with genes at other loci, especially with genes located nearby on the same chromosome. Recombination breaks up this linkage disequilibrium too slowly to avoid genetic hitchhiking, where an allele at one locus rises to high frequency because it is linked to an allele under selection at a nearby locus. This is a problem for population genetic models that treat one gene locus at a time. It can, however, be exploited as a method for detecting the action of natural selection via selective sweeps.
In the extreme case of primarily asexual populations, linkage is complete, and different population genetic equations can be derived and solved, which behave quite differently from the sexual case.[73] Most microbes, such as bacteria, are asexual. The population genetics of microorganisms lays the foundations for tracking the origin and evolution of antibiotic resistance and deadly infectious pathogens. Population genetics of microorganisms is also an essential factor for devising strategies for the conservation and better utilization of beneficial microbes (Xu, 2010).
See also
Notes and references
- ^ a b c Bowler 2003, pp. 325–339
- ^ a b c Larson 2004, pp. 221–243
- ^ Mayr & Provine 1998, pp. 295–298, 416
- ^ a b Provine, W. B. (1988). "Progress in evolution and meaning in life". Evolutionary progress. University of Chicago Press. pp. 49–79.
- ^ a b Provine, William B. (1978). "The role of mathematical population geneticists in the evolutionary synthesis of the 1930s and 1940s". Studies of the History of Biology. 2: 167–192.
- ^ Wright and Fisher on Inbreeding and Random Drift by James F. Crow, published in Genetics Published 2010
- ^ Mayr, Ernst (1988). Toward a New Philosophy of Biology: Observations of an Evolutionist. Cambridge, MA: Belknap Press of Harvard University Press. p. 402. ISBN 0-674-89665-3.
{{cite book}}: Invalid|ref=harv(help) - ^ Mayr & Provine 1998, pp. 338–341
- ^ Jurka, Jerzy, Weidong Bao, Kenji K. Kojima; Bao; Kojima (September 2011). "Families of transposable elements, population structure and the origin of species". Biology Direct. 6: 44. doi:10.1186/1745-6150-6-44. PMC 3183009. PMID 21929767.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Lynch, Michael, John S. Conery; Conery (2003). "The origins of genome complexity". Science. 302 (5649): 1401–1404. Bibcode:2003Sci...302.1401L. doi:10.1126/science.1089370. PMID 14631042.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Postlethwalt, John (2009). Modern Biology. Holt, Rinehart and Winston. p. 317.
- ^ Hartl, Daniel (2007). Principles of Population Genetics. Sinauer Associates. p. 95. ISBN 978-0-87893-308-2.
- ^ JBS Haldane (1927). "A Mathematical Theory of Natural and Artificial Selection, Part V: Selection and Mutation". Mathematical Proceedings of the Cambridge Philosophical Society. 23 (7): 838–844. Bibcode:1927PCPS...23..838H. doi:10.1017/S0305004100015644.
- ^ Orr, H. A. (2010). "The population genetics of beneficial mutations". Philosophical Transactions of the Royal Society B: Biological Sciences. 365 (1544): 1195–1201. doi:10.1098/rstb.2009.0282.
- ^ Hermisson J, Pennings PS; Pennings (2005). "Soft sweeps: molecular population genetics of adaptation from standing genetic variation". Genetics. 169 (4): 2335–2352. doi:10.1534/genetics.104.036947. PMC 1449620. PMID 15716498.
- ^ Ewens W.J. (2004). Mathematical Population Genetics (2nd Edition). Springer-Verlag, New York. ISBN 0-387-20191-2.
- ^ Masel J (2011). "Genetic drift". Current Biology. 21 (20): R837–R838. doi:10.1016/j.cub.2011.08.007. PMID 22032182.
- ^ Futuyma, Douglas (1998). Evolutionary Biology. Sinauer Associates. p. Glossary. ISBN 0-87893-189-9.
- ^ Avers, Charlotte (1989). "Process and Pattern in Evolution". Oxford University Press.
{{cite journal}}: Cite journal requires|journal=(help); Invalid|ref=harv(help) - ^ Futuyma, Douglas (1998). Evolutionary Biology. Sinauer Associates. p. 320. ISBN 0-87893-189-9.
- ^ Gillespie JH (2000). "Genetic Drift in an Infinite Population: The Pseudohitchhiking Model". Genetics. 155 (2): 909–919. PMC 1461093. PMID 10835409.
- ^ Provine, William B. The "Random Genetic Drift" Fallacy. CreateSpace.
- ^ Wahl L.M. (2011). "Fixation when N and s Vary: Classic Approaches Give Elegant New Results". Genetics. 188 (4): 783–785. doi:10.1534/genetics.111.131748. PMC 3176088. PMID 21828279.
- ^ Nicholas H. Barton, Derek E. G. Briggs, Jonathan A. Eisen, David B. Goldstein, Nipam H. Patel (2007). Evolution. Cold Spring Harbor Laboratory Press. p. 417. ISBN 0-87969-684-2.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Sawyer SA, Parsch J, Zhang Z, Hartl DL; Parsch; Zhang; Hartl (2007). "Prevalence of positive selection among nearly neutral amino acid replacements in Drosophila". Proc. Natl. Acad. Sci. U.S.A. 104 (16): 6504–10. Bibcode:2007PNAS..104.6504S. doi:10.1073/pnas.0701572104. PMC 1871816. PMID 17409186.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Hastings, P J; Lupski, JR; Rosenberg, SM; Ira, G (2009). "Mechanisms of change in gene copy number". Nature Reviews. Genetics. 10 (8): 551–564. doi:10.1038/nrg2593. PMC 2864001. PMID 19597530.
{{cite journal}}: Invalid|ref=harv(help) - ^ Sean B. Carroll; Jennifer K. Grenier; Scott D. Weatherbee (2005). From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Second Edition. Oxford: Blackwell Publishing. ISBN 1-4051-1950-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Harrison P, Gerstein M; Gerstein (2002). "Studying genomes through the aeons: protein families, pseudogenes and proteome evolution". J Mol Biol. 318 (5): 1155–74. doi:10.1016/S0022-2836(02)00109-2. PMID 12083509.
{{cite journal}}: Invalid|ref=harv(help) - ^ Orengo CA, Thornton JM; Thornton (2005). "Protein families and their evolution-a structural perspective". Annu. Rev. Biochem. 74: 867–900. doi:10.1146/annurev.biochem.74.082803.133029. PMID 15954844.
{{cite journal}}: Invalid|ref=harv(help) - ^ Long M, Betrán E, Thornton K, Wang W; Betrán; Thornton; Wang (November 2003). "The origin of new genes: glimpses from the young and old". Nat. Rev. Genet. 4 (11): 865–75. doi:10.1038/nrg1204. PMID 14634634.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Wang M, Caetano-Anollés G; Caetano-Anollés (2009). "The evolutionary mechanics of domain organization in proteomes and the rise of modularity in the protein world". Structure. 17 (1): 66–78. doi:10.1016/j.str.2008.11.008. PMID 19141283.
{{cite journal}}: Invalid|ref=harv(help) - ^ Bowmaker JK (1998). "Evolution of colour vision in vertebrates". Eye (London, England). 12 (Pt 3b): 541–7. doi:10.1038/eye.1998.143. PMID 9775215.
{{cite journal}}: Invalid|ref=harv(help) - ^ Gregory TR, Hebert PD; Hebert (1999). "The modulation of DNA content: proximate causes and ultimate consequences". Genome Res. 9 (4): 317–24. doi:10.1101/gr.9.4.317. PMID 10207154.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|doi_brokendate=ignored (|doi-broken-date=suggested) (help) - ^ Hurles M (July 2004). "Gene duplication: the genomic trade in spare parts". PLoS Biol. 2 (7): E206. doi:10.1371/journal.pbio.0020206. PMC 449868. PMID 15252449.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: unflagged free DOI (link) - ^ Liu N, Okamura K, Tyler DM; Okamura; Tyler; Phillips; Chung; Lai (2008). "The evolution and functional diversification of animal microRNA genes". Cell Res. 18 (10): 985–96. doi:10.1038/cr.2008.278. PMC 2712117. PMID 18711447.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Siepel A (October 2009). "Darwinian alchemy: Human genes from noncoding DNA". Genome Res. 19 (10): 1693–5. doi:10.1101/gr.098376.109. PMC 2765273. PMID 19797681.
{{cite journal}}: Invalid|ref=harv(help) - ^ Lynch, M. (2007). "The frailty of adaptive hypotheses for the origins of organismal complexity". PNAS. 104 (suppl. 1): 8597–8604. Bibcode:2007PNAS..104.8597L. doi:10.1073/pnas.0702207104. PMC 1876435. PMID 17494740.
- ^ Smith N.G.C., Webster M.T., Ellegren, H.; Webster; Ellegren (2002). "Deterministic Mutation Rate Variation in the Human Genome". Genome Research. 12 (9): 1350–1356. doi:10.1101/gr.220502. PMC 186654. PMID 12213772.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Petrov DA, Sangster TA, Johnston JS, Hartl DL, Shaw KL; Sangster; Johnston; Hartl; Shaw (2000). "Evidence for DNA loss as a determinant of genome size". Science. 287 (5455): 1060–1062. Bibcode:2000Sci...287.1060P. doi:10.1126/science.287.5455.1060. PMID 10669421.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Petrov DA (2002). "DNA loss and evolution of genome size in Drosophila". Genetica. 115 (1): 81–91. doi:10.1023/A:1016076215168. PMID 12188050.
- ^ Kiontke K, Barriere A , Kolotuev I, Podbilewicz B , Sommer R, Fitch DHA , Felix MA; Barrière; Kolotuev; Podbilewicz; Sommer; Fitch; Félix (2007). "Trends, stasis, and drift in the evolution of nematode vulva development". Current Biology. 17 (22): 1925–1937. doi:10.1016/j.cub.2007.10.061. PMID 18024125.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Braendle C, Baer CF, Felix MA; Baer; Félix (2010). Barsh, Gregory S (ed.). "Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System". PLoS Genetics. 6 (3): e1000877. doi:10.1371/journal.pgen.1000877. PMC 2837400. PMID 20300655.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Palmer, RA (2004). "Symmetry breaking and the evolution of development". Science. 306 (5697): 828–833. Bibcode:2004Sci...306..828P. doi:10.1126/science.1103707. PMID 15514148.
- ^ West-Eberhard, M-J. (2003). Developmental plasticity and evolution. New York: Oxford University Press. ISBN 978-0-19-512235-0.
- ^ Stoltzfus, A and Yampolsky, L.Y. (2009). "Climbing Mount Probable: Mutation as a Cause of Nonrandomness in Evolution". J Hered. 100 (5): 637–647. doi:10.1093/jhered/esp048. PMID 19625453.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yampolsky, L.Y. and Stoltzfus, A (2001). "Bias in the introduction of variation as an orienting factor in evolution". Evol Dev. 3 (2): 73–83. doi:10.1046/j.1525-142x.2001.003002073.x. PMID 11341676.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Haldane, JBS (1933). "The Part Played by Recurrent Mutation in Evolution". American Naturalist. 67 (708): 5–19. doi:10.1086/280465. JSTOR 2457127.
- ^ Protas, Meredith; Conrad, M; Gross, JB; Tabin, C; Borowsky, R (2007). "Regressive evolution in the Mexican cave tetra, Astyanax mexicanus". Current Biology. 17 (5): 452–454. doi:10.1016/j.cub.2007.01.051. PMC 2570642. PMID 17306543.
{{cite journal}}: Invalid|ref=harv(help) - ^ Maughan H, Masel J, Birky WC, Nicholson WL; Masel; Birky Jr; Nicholson (2007). "The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis". Genetics. 177 (2): 937–948. doi:10.1534/genetics.107.075663. PMC 2034656. PMID 17720926.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Masel J, King OD, Maughan H; King; Maughan (2007). "The loss of adaptive plasticity during long periods of environmental stasis". American Naturalist. 169 (1): 38–46. doi:10.1086/510212. PMC 1766558. PMID 17206583.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bertram J (2000). "The molecular biology of cancer". Mol. Aspects Med. 21 (6): 167–223. doi:10.1016/S0098-2997(00)00007-8. PMID 11173079.
{{cite journal}}: Invalid|ref=harv(help) - ^ Sniegowski P, Gerrish P, Johnson T, Shaver A; Gerrish; Johnson; Shaver (2000). "The evolution of mutation rates: separating causes from consequences". BioEssays. 22 (12): 1057–66. doi:10.1002/1521-1878(200012)22:12<1057::AID-BIES3>3.0.CO;2-W. PMID 11084621.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Drake JW, Holland JJ; Holland (1999). "Mutation rates among RNA viruses". Proc. Natl. Acad. Sci. U.S.A. 96 (24): 13910–3. Bibcode:1999PNAS...9613910D. doi:10.1073/pnas.96.24.13910. PMC 24164. PMID 10570172.
{{cite journal}}: Invalid|ref=harv(help) - ^ Holland J, Spindler K, Horodyski F, Grabau E, Nichol S, VandePol S; Spindler; Horodyski; Grabau; Nichol; Vandepol (1982). "Rapid evolution of RNA genomes". Science. 215 (4540): 1577–85. Bibcode:1982Sci...215.1577H. doi:10.1126/science.7041255. PMID 7041255.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Morjan C, Rieseberg L; Rieseberg (2004). "How species evolve collectively: implications of gene flow and selection for the spread of advantageous alleles". Mol. Ecol. 13 (6): 1341–56. doi:10.1111/j.1365-294X.2004.02164.x. PMC 2600545. PMID 15140081.
{{cite journal}}: Invalid|ref=harv(help) - ^ Gravel, S., S. (2012). "Population Genetics Models of Local Ancestry". Genetics. 1202 (2): 4811. arXiv:1202.4811. Bibcode:2012arXiv1202.4811G. doi:10.1534/genetics.112.139808.
- ^ Su H, Qu L, He K, Zhang Z, Wang J, Chen Z, Gu H; Qu; He; Zhang; Wang; Chen; Gu (2003). "The Great Wall of China: a physical barrier to gene flow?". Heredity. 90 (3): 212–9. doi:10.1038/sj.hdy.6800237. PMID 12634804.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Short RV (1975). "The contribution of the mule to scientific thought". J. Reprod. Fertil. Suppl. (23): 359–64. PMID 1107543.
{{cite journal}}: Invalid|ref=harv(help) - ^ Gross B, Rieseberg L; Rieseberg (2005). "The ecological genetics of homoploid hybrid speciation". J. Hered. 96 (3): 241–52. doi:10.1093/jhered/esi026. PMC 2517139. PMID 15618301.
{{cite journal}}: Invalid|ref=harv(help) - ^ Burke JM, Arnold ML; Arnold (2001). "Genetics and the fitness of hybrids". Annu. Rev. Genet. 35: 31–52. doi:10.1146/annurev.genet.35.102401.085719. PMID 11700276.
{{cite journal}}: Invalid|ref=harv(help) - ^ Vrijenhoek RC (2006). "Polyploid hybrids: multiple origins of a treefrog species". Curr. Biol. 16 (7): R245–7. doi:10.1016/j.cub.2006.03.005. PMID 16581499.
{{cite journal}}: Invalid|ref=harv(help) - ^ Wendel J (2000). "Genome evolution in polyploids". Plant Mol. Biol. 42 (1): 225–49. doi:10.1023/A:1006392424384. PMID 10688139.
{{cite journal}}: Invalid|ref=harv(help) - ^ Sémon M, Wolfe KH; Wolfe (2007). "Consequences of genome duplication". Curr Opin Genet Dev. 17 (6): 505–12. doi:10.1016/j.gde.2007.09.007. PMID 18006297.
{{cite journal}}: Invalid|ref=harv(help) - ^ Comai L (2005). "The advantages and disadvantages of being polyploid". Nat. Rev. Genet. 6 (11): 836–46. doi:10.1038/nrg1711. PMID 16304599.
{{cite journal}}: Invalid|ref=harv(help) - ^ Soltis P, Soltis D; Soltis (June 2000). "The role of genetic and genomic attributes in the success of polyploids". Proc. Natl. Acad. Sci. U.S.A. 97 (13): 7051–7. Bibcode:2000PNAS...97.7051S. doi:10.1073/pnas.97.13.7051. PMC 34383. PMID 10860970.
{{cite journal}}: Invalid|ref=harv(help) - ^ Boucher Y, Douady CJ, Papke RT, Walsh DA, Boudreau ME, Nesbo CL, Case RJ, Doolittle WF; Douady; Papke; Walsh; Boudreau; Nesbø; Case; Doolittle (2003). "Lateral gene transfer and the origins of prokaryotic groups". Annu Rev Genet. 37: 283–328. doi:10.1146/annurev.genet.37.050503.084247. PMID 14616063.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Walsh T (2006). "Combinatorial genetic evolution of multiresistance". Curr. Opin. Microbiol. 9 (5): 476–82. doi:10.1016/j.mib.2006.08.009. PMID 16942901.
{{cite journal}}: Invalid|ref=harv(help) - ^ Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T; Nikoh; Ijichi; Shimada; Fukatsu (2002). "Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect". Proc. Natl. Acad. Sci. U.S.A. 99 (22): 14280–5. Bibcode:2002PNAS...9914280K. doi:10.1073/pnas.222228199. PMC 137875. PMID 12386340.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Sprague G (1991). "Genetic exchange between kingdoms". Curr. Opin. Genet. Dev. 1 (4): 530–3. doi:10.1016/S0959-437X(05)80203-5. PMID 1822285.
{{cite journal}}: Invalid|ref=harv(help) - ^ Gladyshev EA, Meselson M, Arkhipova IR; Meselson; Arkhipova (May 2008). "Massive horizontal gene transfer in bdelloid rotifers". Science. 320 (5880): 1210–3. Bibcode:2008Sci...320.1210G. doi:10.1126/science.1156407. PMID 18511688.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - ^ Baldo A, McClure M; McClure (1 September 1999). "Evolution and horizontal transfer of dUTPase-encoding genes in viruses and their hosts". J. Virol. 73 (9): 7710–21. PMC 104298. PMID 10438861.
{{cite journal}}: Invalid|ref=harv(help) - ^ Poole A, Penny D; Penny (2007). "Evaluating hypotheses for the origin of eukaryotes". BioEssays. 29 (1): 74–84. doi:10.1002/bies.20516. PMID 17187354.
{{cite journal}}: Invalid|ref=harv(help) - ^ Michael M. Desai, Daniel S. Fisher; Fisher (2007). "Beneficial Mutation Selection Balance and the Effect of Linkage on Positive Selection". Genetics. 176 (3): 1759–1798. doi:10.1534/genetics.106.067678. PMC 1931526. PMID 17483432.
Bibliography
- J. Beatty. "The synthesis and the synthetic theory" in Integrating Scientific Disciplines, edited by W. Bechtel and Nijhoff. Dordrecht, 1986.
- Bowler, Peter J. (2003). Evolution : the history of an idea (3rd ed.). Berkeley: University of California Press. ISBN 978-0-520-23693-6.
{{cite book}}: Invalid|ref=harv(help) - Buston, PM; Pilkington, J. G. (2007). "Are clownfish groups composed of close relatives? An analysis of microsatellite DNA vraiation in Amphiprion percula". Molecular Ecology. 12 (3): 733–742. doi:10.1046/j.1365-294X.2003.01762.x. PMID 12675828.
{{cite journal}}: Invalid|ref=harv(help); Unknown parameter|displayauthors=ignored (|display-authors=suggested) (help) - Luigi Luca Cavalli-Sforza. Genes, Peoples, and Languages. North Point Press, 2000.
- Luigi Luca Cavalli-Sforza et al. The History and Geography of Human Genes. Princeton University Press, 1994.
- James F. Crow and Motoo Kimura. Introduction to Population Genetics Theory. Harper & Row, 1972.
- Warren J Ewens. Mathematical Population Genetics. Springer-Verlag New York, Inc., 2004. ISBN 0-387-20191-2
- John H. Gillespie Population Genetics: A Concise Guide, Johns Hopkins Press, 1998. ISBN 0-8018-5755-4.
- Richard Halliburton. Introduction to Population Genetics. Prentice Hall, 2004
- Daniel Hartl. Primer of Population Genetics, 3rd edition. Sinauer, 2000. ISBN 0-87893-304-2
- Daniel Hartl and Andrew Clark. Principles of Population Genetics, 3rd edition. Sinauer, 1997. ISBN 0-87893-306-9.
- Larson, Edward J. (2004). Evolution : the remarkable history of a scientific theory (Modern Library ed.). New York: Modern Library. ISBN 978-0-679-64288-6.
{{cite book}}: Invalid|ref=harv(help) - Richard C. Lewontin. The Genetic Basis of Evolutionary Change. Columbia University Press, 1974.
- William B. Provine. The Origins of Theoretical Population Genetics. University of Chicago Press. 1971. ISBN 0-226-68464-4.
- Repaci, V; Stow AJ, Briscoe DA (2007). "Fine-scale genetic structure, co-founding and multiple mating in the Australian allodapine bee (Ramphocinclus brachyurus". Journal of Zoology. 270 (4): 687–691. doi:10.1111/j.1469-7998.2006.00191.x.
{{cite journal}}: Invalid|ref=harv(help) - Spencer Wells. The Journey of Man. Random House, 2002.
- Spencer Wells. Deep Ancestry: Inside the Genographic Project. National Geographic Society, 2006.
- Cheung, KH; Osier MV, Kidd JR, Pakstis AJ, Miller PL, Kidd KK (2000). "ALFRED: an allele frequency database for diverse populations and DNA polymorphisms". Nucleic Acids Research. 28 (1): 361–3. doi:10.1093/nar/28.1.361. PMC 102486. PMID 10592274.
{{cite journal}}: Invalid|ref=harv(help)CS1 maint: multiple names: authors list (link) - Xu, J. Microbial Population Genetics. Caister Academic Press, 2010. ISBN 978-1-904455-59-2
External links
- Population Genetics Tutorials
- The ALlele FREquency Database at Yale University
- EHSTRAFD.org - Earth Human STR Allele Frequencies Database
- History of population genetics
- How Selection Changes the Genetic Composition of Population, video of lecture by Stephen C. Stearns (Yale University)
- National Geographic: Atlas of the Human Journey (Haplogroup-based human migration maps)
- Monash Virtual Laboratory - Simulations of habitat fragmentation and population genetics online at Monash University's Virtual Laboratory.




